3D FIB/SEM acquisition and reconstruction of polymeric membranes for microalgae filtration applications
- Abstract number
- 511
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.511
- Corresponding Email
- [email protected]
- Session
- PSA.8 - Microscopy in industrial applications
- Authors
- Ms Hélène Roberge (1, 2), Pr Philippe Moreau (1), Dr Estelle Couallier (2), Dr Patricia Abellan (1)
- Affiliations
-
1. Université de Nantes, CNRS, Institut des Matériaux Jean Rouxel, IMN
2. Université de Nantes, CNRS, ONIRIS, Laboratoire de Génie des Procédés, Environnement et Agroalimentaire, GEPEA
- Keywords
3D characterization, 3D FIB/SEM, Filtration membrane, Polymer sample
- Abstract text
Membrane filtration processes allow to concentrate, separate and purify the components of a complex mixture in liquid phase. The main role of the membrane is to allow the permeation of one element, while blocking others because of size or charge exclusion. Typically, these processes are used in water treatment and agribusiness industries. Recently, they have been adapted for microalgae valorisation. Indeed, microalgae can produce lipids, proteins or polysaccharides that can be used in a vast array of areas, such as pharmaceutical industry (antibiotics), cosmetics (antioxydants), food supplements production (proteins rich in several amino acids) and in the biofuel industry as biodiesel (triglycerides). Ultrafiltration and microfiltration have been studied to separate lipids and proteins from microalgae, for which porous polymer membranes are used. The accumulation of biomolecules at the surface and in the porous medium, termed fouling, is a major operational challenge and a well-known drawback in membrane filtration. The prevention of fouling requires a detailed characterization of the structure of filtration membranes as well as their interaction with the different target biomolecules.
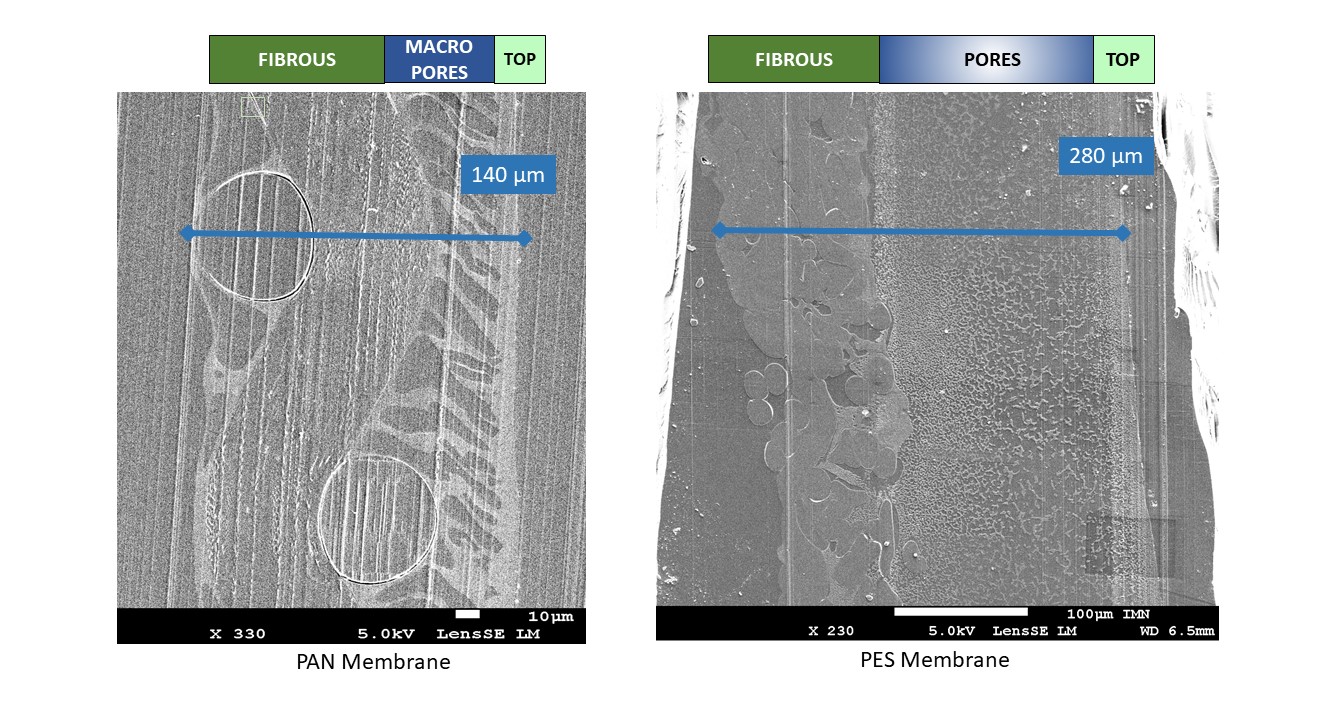
Two types of organic polymer membranes have been tested in the GEPEA laboratory for the fractionation of biomolecules from microalgae: PAN (polyacrylonitrile, 30 nm, Orelis) and PES (0.1 μm polyethersulfone, Koch) [1] [2]. They consist of a thin active layer, which has the main role in the filtration selectivity, deposited on a macroporous structure allowing high permeability and mechanical resistance. According to the suppliers, the pores at the surface are of around 30 nm in size for the PAN and 100 nm for the PES. However, there are evidences that they have a more complex porous structure which is not yet well understood. Some hypotheses have been made on the structure of membrane fouling (pore blockage, pore constriction, cake at the membrane surface) using macroscopic data (water flux, contact angles, zeta potential) but their direct characterization remains a challenge.
Generally, membrane pores distributions are characterized with SEM (Scanning electron microscopy) at the surface and within the bulk (membranes are frozen in liquid nitrogen and fractured to access their interior) [3]. Identification of the active layer and the characterization of its pores with (sub)nanometer resolution can be achieved using TEM (transmission electron microscopy) and STEM (Scanning transmission electron microscopy), for which sample preparation is key. However, pores are 3D elements and difficult to characterize on 2D basis only. It is now possible to characterize porous materials in 3D with a few nanometers resolution and wide field of views using FIB (focused ion beam) coupled with SEM. [4].
In this presentation, (S)TEM and FIB/SEM are employed to characterize the complex structure of PAN and PES ultra and microfiltration polymer membranes at relevant length scales (Figure 1 shows the different mesoscopic layers forming the PES and PAN membranes). We performed a structural 3D characterization, aimed at the identification and detailed characterization of the active layer and the nanometric pores involved in fouling, by FIB/SEM. This study is particularly challenging due to the fact that polymer membranes are amorphous, present little contrast and a
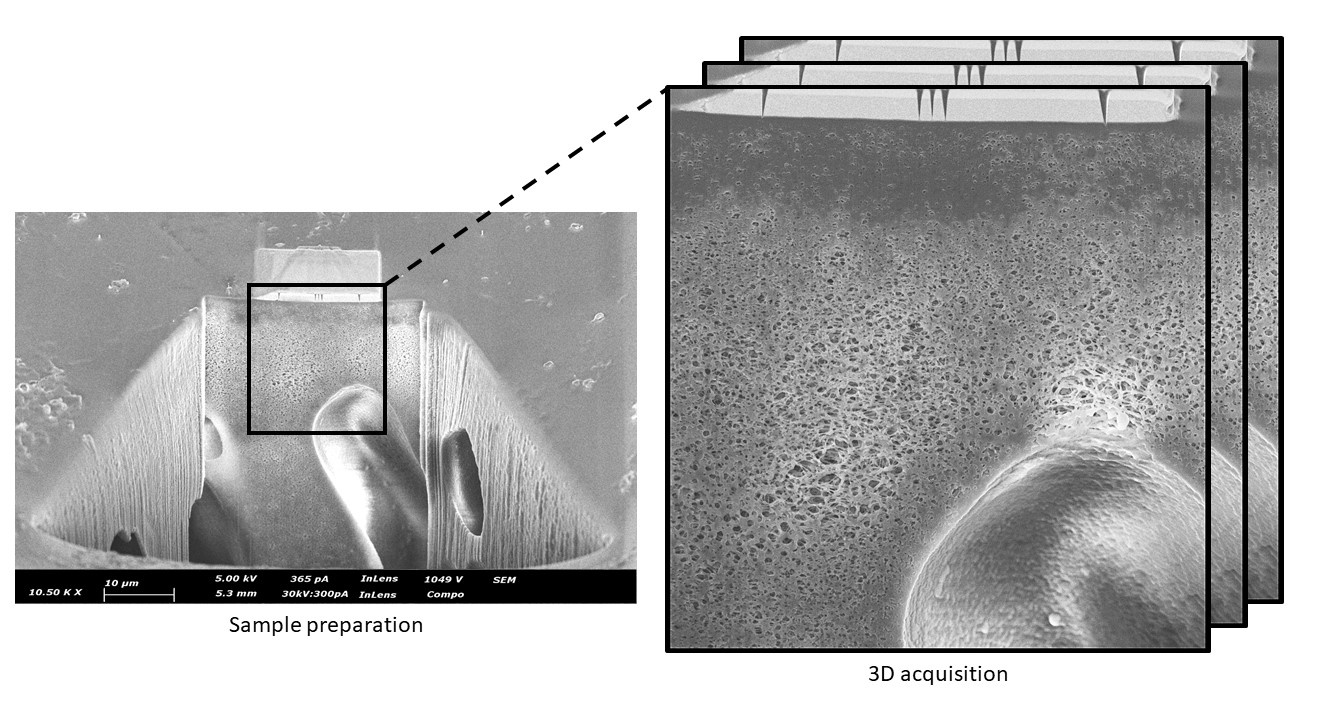
strong sensitivity to the electron and ion beams and thus, experimental parameters must be optimized for each type of experiment. Both pristine membranes and fouled membranes will be presented. The sample preparation as well as the optimization of acquisition and reconstruction 3D FIB/SEM parameters will be described (Figure 2 shows a PAN membrane after 3D FIB/SEM sample preparation). [5]Figure 1: Observation by SEM of embedded PAN and PES filtration membrane
Figure 2: Sample preparation for 3D FIB/SEM (PAN polymer membrane)
- References
[1] Clavijo Rivera, Erika, Liliana Villafaña-López, Shuli Liu, R. Vinoth Kumar, Michèle Viau, Patrick Bourseau, Cécile Monteux, Matthieu Frappart, et Estelle Couallier. « Cross-Flow Filtration for the Recovery of Lipids from Microalgae Aqueous Extracts: Membrane Selection and Performances ». Process Biochemistry 89 (1 février 2020): 199-207. https://doi.org/10.1016/j.procbio.2019.10.016.
[2] Villafaña-López, Liliana, Erika Clavijo Rivera, Shuli Liu, Estelle Couallier, et Matthieu Frappart. « Shear-Enhanced Membrane Filtration of Model and Real Microalgae Extracts for Lipids Recovery in Biorefinery Context ». Bioresource Technology 288 (1 septembre 2019): 121539. https://doi.org/10.1016/j.biortech.2019.121539.
[3] Rahimpour, A., et S. S. Madaeni. « Polyethersulfone (PES)/Cellulose Acetate Phthalate (CAP) Blend Ultrafiltration Membranes: Preparation, Morphology, Performance and Antifouling Properties ». Journal of Membrane Science 305, no 1 (15 novembre 2007). https://doi.org/10.1016/j.memsci.2007.08.030.
[4] Sundaramoorthi, Ganesh, Markus Hadwiger, Mohamed Ben-Romdhane, Ali R. Behzad, Poornima Madhavan, et Suzana P. Nunes. « 3D Membrane Imaging and Porosity Visualization ». Industrial & Engineering Chemistry Research 55, no 12 (30 mars 2016). https://doi.org/10.1021/acs.iecr.6b00387.
[5] The authors would like to thank the financial support by the NExT initiative through national funding by the French National Research Agency (ANR) under the Programme d'investissements d'Avenir (with reference ANR-16-IDEX-0007). The project also receives financial support from the Pays de la Loire Region and Nantes Métropole.