A new approach to measuring subcellular elemental distributions using analytical electron microscopy.
- Abstract number
- 411
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.411
- Corresponding Email
- [email protected]
- Session
- PST.4 - Spectroscopies in Electron, X-ray and Ion Microscopy
- Authors
- Ms Alexandra Sheader (2), Ms Christina Ferguson (3), Professor Roland Fleck (1), Dr Sarah Flatters (3), Professor Peter Nellist (2)
- Affiliations
-
1. Centre for Ultrastructural Imaging, King’s College London
2. Department of Materials, University of Oxford
3. Wolfson Centre for Age-Related Diseases, Institute of Psychiatry, Psychology and Neuroscience, King’s College London
- Keywords
cross-section, EDX, EELS, HAADF, life sciences, STEM,
- Abstract text
In this work we demonstrate how energy dispersive x-ray (EDX) and electron energy loss spectroscopy (EELS) partial scattering cross sections may be used for quantification of elements of biological significance in tissue samples. We demonstrate how measurement of Na, K and Ca concentrations can be made samples of freeze-dried cryosectioned peripheral nerve at high resolution and with high sensitivity.
Partial scattering cross-sections are used throughout the physical sciences to measure interaction processes. In the electron microscope, both energy-dispersive x-ray (EDX) and electron energy loss spectra (EELS) can be interpreted in terms of appropriate cross-sections [1,2]. The unit of ‘barns’ describes an effective area (with 1barn equivalent to 10-28m2), and these cross-section measurements can account for many key parameters in quantifying analytical spectra, including detector geometry and efficiency. Such measurements also allow for benchmarking instrumental configurations against each other. This approach has been shown to offer advantages over conventional quantification techniques in examination of bimetallic catalytic nanoparticles [3]. However, the extent to which it can be implemented in the life sciences has remained unexplored.
High resolution measurement of elemental distributions is an important challenge in many biological fields. The attempts of established and emerging techniques to address this problem typically experience difficulty with providing either high specificity, sensitivity or spatial resolution [4]. In this presentation, we will demonstrate an alternative approach based on elastic and inelastic scattering processes in the scanning transmission electron microscope (STEM). We present a new method for making concentration measurements of sodium, potassium and calcium in freeze-dried cryosectioned tissue samples by using simultaneous collection of EDX, EELS and high-angle annular dark field (HAADF) signal from known nanoparticle standards (see Figure 1). We compare the detection efficiency of different microscope configurations and explore the impact of this on achieving biologically relevant results.
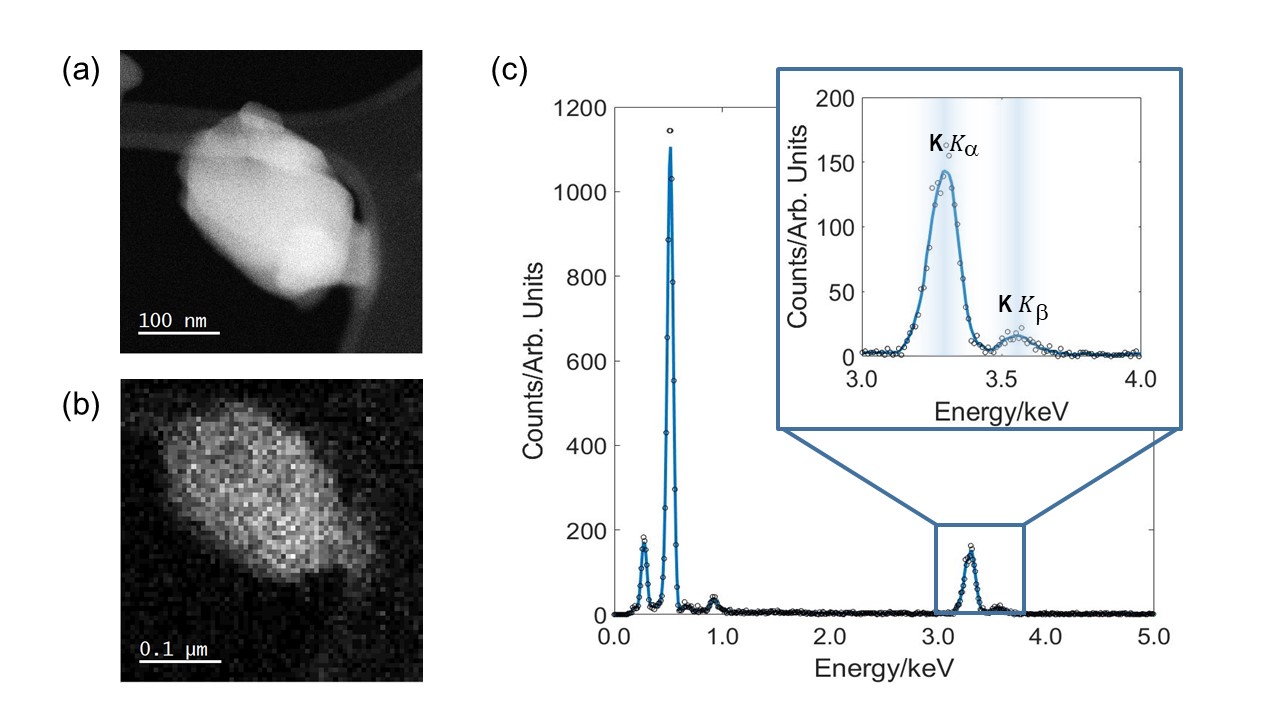
Figure 1: EDX data acquired from BK3O3 nanoparticle. (a) HAADF image used for mass quantification. (b) EDX spectral image. (c) EDX data obtained from particle in (b). Inset shows the potassium Kα and Kβ peaks at around 3.3keV and 3.6keV respectively.
Peripheral rat nerve is used as a test case to show how it is possible to discriminate between difference cell types at the microscope and make concentration measurements on a neuron-by-neuron or even subcellular basis. The implications of sample preparation on the fate of intracellular elemental concentrations will be discussed alongside the inherent challenges presented by common peak overlaps in EDX, and the significant carbon background in EELS. As with atom counting techniques developed for HAADF imaging [5], we will show how EDX and EELS partial scattering cross-section values can be converted to number of atoms. This value may subsequently be equivalently given in more familiar units of mmol/kg dry weight. In some cases, this can be performed at spatial resolutions of only a few nanometres (see Figure 2). We will identify promising applications of this cross-section based approach, including some results from our own laboratories.
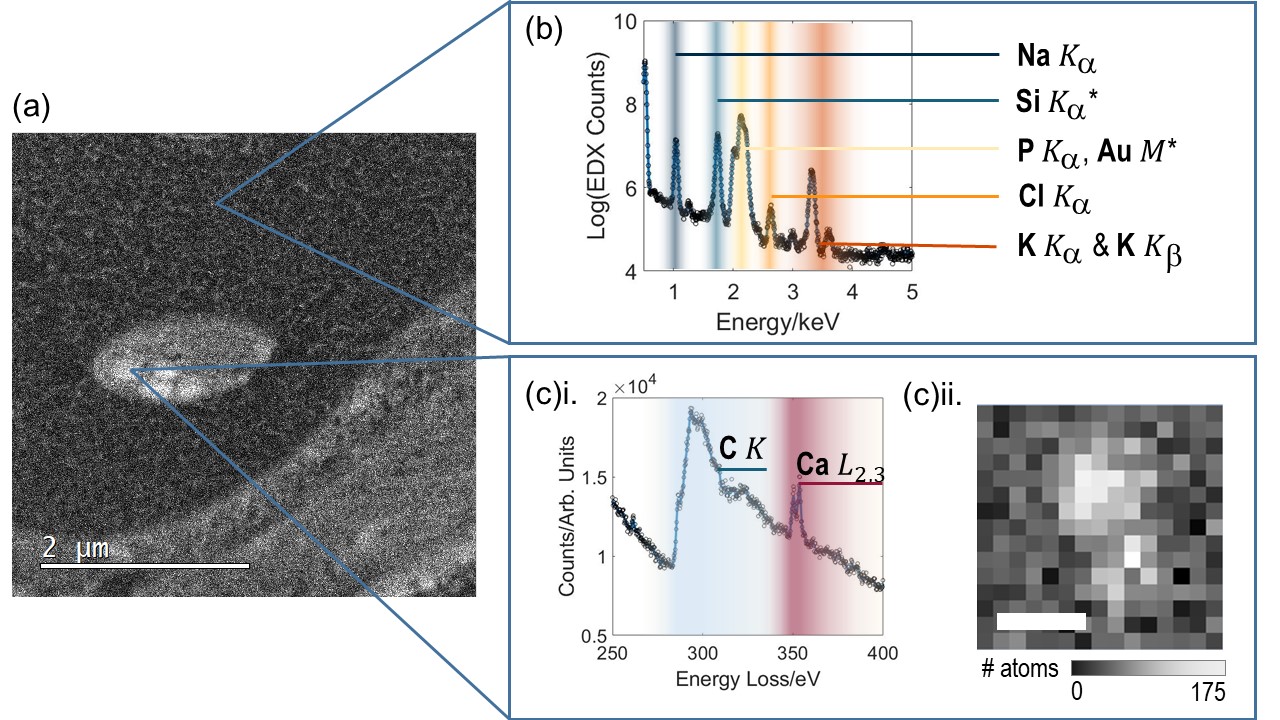
Figure 2: Intracellular elemental measurement using EDX and EELS. (a) shows a HAADF image of an intercellular compartment with visible calcium phosphate storage granules. (b) EDX acquired from cytosol inside a neuron section. Peaks labelled with (*) indicate system peaks. (c)i. Example of EELS data from a mitochondrial calcium phosphate store, showing both C and Ca edges. (c)ii. shows an EELS spectral image from one such CaPO store, with EELS Ca L2,3 intensity converted to number of atoms per pixel. Scalebar 20nm. Challenges for quantifying using both EDX and EELS are readily apparent but nevertheless both types of spectra can be converted into mmol/kg dry weight by integrating over a region of interest.
- References
[1] MacArthur, K. et al. Quantitative energy-dispersive x-ray analysis of catalyst nanoparticles using a partial cross section approach. Microscopy and Microanalysis, 22(1), 71-81 (2016).
[2] Hofer, F. Determination of inner-shell cross-sections for EELS-quantification. Microsc. Microanal. Microstruct., 2 (2-3), 215-230 (1991).
[3] Varambhia, AM. et al. Determining EDS and EELS partial cross-sections from multiple calibration standards to accurately quantify bi-metallic nanoparticles using STEM. Micron, 113, 69-82 (2018).
[4] Decelle, J. et al. Subcellular Chemical Imaging: New Avenues in Cell Biology. Trends in Cell Biol. In press (2020)
[5] Sheader AA. et al. Observation of metal nanoparticles at atomic resolution in Pt‐based cancer chemotherapeutics. Journal of Microscopy, 270, 92-97 (2018).
[6] The authors acknowledge use of characterisation facilities within the David Cockayne Centre for Electron Microscopy, Department of Materials, University of Oxford and in particular the EPSRC (EP/K040375/1 “South of England Analytical Electron Microscope”) and additional instrument provision from the Henry Royce Institute (Grant reference EP/R010145/1). AAS acknowledges the support of EPSRC Doctoral Training Partnership (Project References 1801634 and EP/N509711/1).