Atom Probe Characterisation of Hydroxyapatite Nanoparticle
- Abstract number
- 948
- Event
- Virtual Early Career European Microscopy Congress 2020
- Presentation Form
- Submitted Oral
- DOI
- 10.22443/rms.emc2020.948
- Corresponding Email
- [email protected]
- Session
- PSA.5 - Nanoparticles & Catalysts
- Authors
- Dr. Yi-Sheng Chen (1, 3), Daniel Mosiman (1, 2), Dr. Limei Yang (1), Professor Benito Mariñas (2), Professor Julie Cairney (1, 3)
- Affiliations
-
1. Australian Centre for Microscopy and Microanalysis, The University of Sydney
2. Department of Civil and Environmental Engineering, University of Illinois at Urbana–Champaign
3. School of Aerospace, Mechanical and Mechatronic Engineering, The University of Sydney
- Keywords
atom probe tomography, hydroxyapatite, materials characterisation, nanoparticle
- Abstract text
Fluorosis is a disease known for its major effects in teeth discoloring, arthritic joint pain, and in some cases crippling due to the excess intake of fluoride from drinking water. It is a serious problem for people in low-income, rural areas where excess fluoride is endemic, because they lack access to alternative water sources and cannot afford standard treatment options [1]. This problem can be addressed via using calcium hydroxyapatite (HAP, Ca5(PO4)3OH) nanoparticles (NPs) to exchange the fluorine ion (F-) in water with the hydroxide ion (OH-) of HAP to form calcium fluorapatite (FAP, Ca5(PO4)3F), which is thermodynamically more stable than HAP. This approach has the advantages of cost-effectiveness in mass production, simplicity of operation, and high selectivity for fluoride [1]. However, in practice this technique has only achieved F- uptake at an order of magnitude below its theoretical capacity, and hence, a better understanding of the fundamental F- uptake mechanism(s) of HAP NPs is required for better design/synthesis/manufacturing strategies to produce the future products.
To investigate these mechanism(s), characterization techniques capable of examining individual HAP NPs to concurrently determine their structure and chemical composition are a prerequisite. Transmission electron microscopy (TEM) has been successfully applied to observe HAP NPs at the atomic level and can verify its hexagonal P63/m crystal structure [2]. However, due to the high susceptibility of HAP NPs to electron beam damage, TEM has not yielded convincing data concerning nanometer-level distribution of F- ions on and in individual HAP NPs. To address this, atom probe tomography (APT) is capable because of its advantageous spatial resolution for nanoscale features and chemical precision based upon its time-of-flight spectroscopic configuration. However, progress has been limited by the strict geometry requirement of the APT sample – an ultra-sharp needle with a 50 nm radius – within which individual HAP NPs (rods averaging 60 nm long by 20 nm dia.) must be fixed. To achieve this sample configuration, HAP NPs must be well dispersed on a substrate and encapsulated by a suitable material, followed by focused ion beam (FIB) assisted lift out and needle milling, as demonstrated in [3]. Also, consideration of the compatibility of the encapsulation/substrate materials with HAP to minimize the extent of peak overlaps in APT mass spectra must be considered to acquire high quality data [4].
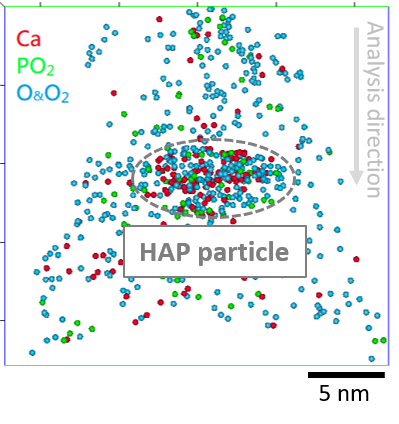
To address these considerations, a recipe was developed to more evenly disperse HAP NPs in methanol rather than acetone, with no ligand addition to retain the virginity of the material composition, as shown in Figure 1(a) and (b), respectively. HAP encapsulation using a gold substrate and a gold coating was carried out prior to FIB sample preparation, yielding a mass spectrum that contains data without peak overlaps between the encapsulating material and the primary peaks of HAP, as shown in Figure 2(a). The resulting atom probe observation of a HAP NP at atomic scale is shown in Figure 2(b) which suggests the applicability of the proposed protocol to provide insights into the fluoride uptake mechanism(s) of HAP NPs.
Figure 1(a)
(b)
Figure 1. TEM images of the HAP particles dispersed in (a) methanol and (b) acetone where the former shows improved dispersion of particles
Figure 2(a)
(b)
Figure 2. (a) Mass spectrum of an atom probe specimen of gold incorporating HAP a particle. (b) 5-nm slice of the 3D atom map of HAP related ions (Ca, PO2, O and O2) showing the presence of HAP particle within the dataset.
- References
[1] J.K.Fawell et al. ‘Fluoride in drinking-water’, WHO drinking water quality series, (2006)
[2] L. Bertinetti et al., Journal of Physical Chemistry C 111 (2017), p.4027-4035
[3] P. Felfer et al., Angewandte Chemie International Edition 53 (2014), p.11190-11193.
[4] L. M. Gordon et al., ACS Nano 6 (2012), p.10667-10675