Atomic scale understanding of ion exchange in atomically thin clays and micas by STEM
- Abstract number
- 984
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.984
- Corresponding Email
- [email protected]
- Session
- PSA.1 - 1D & 2D Materials
- Authors
- Dr Yichao Zou (1), Mr Lucas Moggs (1), Dr Nicholas Clark (1), Dr Yi-Chi Wang (1), Mr David Hopkinson (1), Prof Andre Geim (1), Dr Marcelo Lozada-Hidalgo (1), Prof Sarah Haigh (1)
- Affiliations
-
1. University of Manchester
- Keywords
scanning transmission electron microscopy, 2D materials, geology, ion exchange, materials science
- Abstract text
Introduction
Ion exchangers are non-soluble materials that contain exchangeable ions. These ions are easily substituted with other ions of the same polarity when the material is immersed in suitable electrolytes [1]. Layer structured clays and micas are well-known ion exchangers. They consist of aluminosilicate layers which are covered or connected with cations such as K+ and Mg2+. These native cations can be exchanged for other ions, e.g. H+, Li+ or Cs+, which adsorb both on the surface and interlayer space of the aluminosilicate layers. It has been recently shown that when exfoliated down to nanometer thickness, proton-exchanged clays and micas become excellent proton conducting membranes, displaying high conductivity at elevated temperatures that has remained largely inaccessible with current materials [1]. Ion uptake experiments have demonstrated exceptionally fast ion exchange for clay (vermiculite) and mica (muscovite, biotite) samples that have been exfoliated down to few layer and mono-layer. In this work, by imaging ion-exchanged flakes of different thicknesses, we demonstrate new insight into the mechanism behind this fast uptake – in particular the importance of interlayer space, edges, step edges, and surface.
Materials and Methods
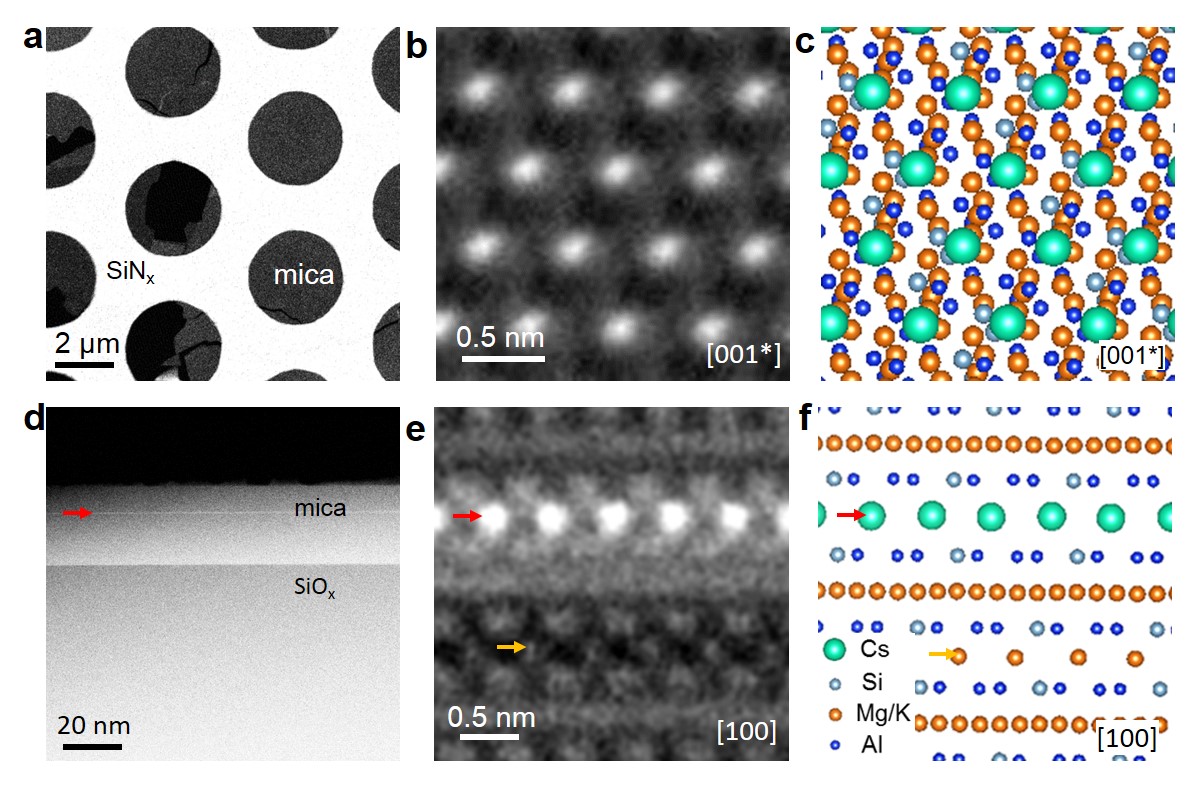
For plan-view STEM specimens (Fig. 1a-c), preparation is performed by first identifying mechanically exfoliated atomically thin mica/clay flakes using optical microscopy and atomic force microscopy. These flakes were then transferred over holes in home-made SiNx membrane supported grids (Fig. 1a) and cleaned using acetone, isopropyl alcohol and de-ionized water. The samples were then ion exchanged by immersing the whole TEM grid assembly into a CsNO3 water solution for the desired time length, variable from one second to several months. The as-exchanged samples are then washed using de-ionized water and ethanol, and dried by nitrogen gas prior to STEM analysis.
For cross sectional STEM samples, we applied focused ion beam milling (FIB) using a FEI Helios 660 FIB microscope, conducted on flakes that are exfoliated onto SiOx/Si substrate and subjected to the ion exchange process mentioned above (Fig. 1d-f). Standard milling protocols were used to cut the lamella free from the substrate and transfer it to a pillar of an OmniProbe™ Cu TEM support grid, followed by 30 kV, 16 kV, 5 kV and 2 kV ion beam milling and polishing to electron transparency.
STEM imaging was performed using a probe-side corrected FEI Titan G2 80-200 S/TEM ChemiSTEM microscope and a JEOL ARM300CF double aberration corrected microscope with a cold FEG electron source. For high resolution annular dark field (ADF) STEM imaging, the Titan was operated at 200 kV with a 21 mrad convergence semi-angle, a 48-191 mrad ADF collection angle. The JEOL ARM300CF was operated at an accelerating voltage of 80 kV, with a beam convergence semi-angle of 31 mrad with an ADF collection angle of 68-206 mrad. The probe current used in the experiments is below 20 pA with a small total dose (< 3 x 104 e·A-2) to avoid beam damage.
Results/Discussion
Fig. 1 displays ADF-STEM characterization for interlayer Cs+ ion exchange in clays and micas, using both plan-view (Fig. 1a-c) and cross-sectional imaging (Fig. 1d-f). In the atomic-number contrast ADF images, Cs+ (ZCs=55) occupied columns display bright contrast, which can be easily distinguished from the native ions in clay and micas which are composed of light elements (ZO=6, ZMg=12, ZAl=13, ZSi=14, ZK=19). By taking advantage of such contrast, as well as the thin feature of the flakes, plan-view imaging can illustrate the lateral distribution of exchanged Cs+ ions. The in-plane arrangement of exchanged Cs+ are found to be ordered with a quasi-hexagonal symmetry, as shown in Fig. 1b,c. The out of plane distribution of the Cs ions in biotite mica flakes is found to segregate onto specific atomic layers, as shown in Fig. 1d-e.
Fig. 1 ADF-STEM characterizations for interlayer Cs exchange in biotite flakes. (a) A plan-view image showing a few-layer Cs-exchanged biotite mica flake suspended over a SiNx TEM grid. (b) Corresponding high resolution ADF STEM image taken along [001*] axis. (c) Corresponding plan-view atomic model. (d) Over-view cross-sectional image showing a Cs exchanged biotite mica flake on SiOx/Si substrate. The atomic plane that Cs+ ion occupied is denoted by a red arrow. (e) Corresponding high resolution ADF STEM image, where both the exchanged ion (red arrow) and the native ions (yellow arrow) can be imaged. (f) Corresponding cross-sectional atomic model.
Conclusion
The in-plane and out-of-plane distribution of the exchanged ions in mechanically exfoliated few layer clays and micas can be investigated at the atomic scale by STEM imaging. This approach complements bulk analysis techniques [2] by allowing new understanding of the ion exchange behaviour at surface, edges and step edges sites. Here we show that surfaces and defects are more favourable for ion exchange compared to the interlayer space in a bulk. This results in the observed ultrafast ion exchange capability of atomically thin crystals, providing potential for improved performance in membrane applications.
- References
1. Mogg, L.; Hao, G. P.; Zhang, S.; Bacaksiz, C.; Zou, Y. C.; Haigh, S. J.; Peeters, F. M.; Geim, A. K.; Lozada-Hidalgo, M., Atomically thin micas as proton-conducting membranes. Nature Nanotechnology 2019, 14, 962–966.
2. Fuller, A. J.; Shaw, S.; Ward, M. B.; Haigh, S. J.; Mosselmans, J. F. W.; Peacock, C. L.; Stackhouse, S.; Dent, A. J.; Trivedi, D.; Burke, I. T., Caesium incorporation and retention in illite interlayers. Applied Clay Science 2015, 108, 128-134.