Biogenic Mn(II)-coated UO2 nanoparticles: TEM experiment and modeling
- Abstract number
- 845
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.845
- Corresponding Email
- [email protected]
- Session
- PSA.6 - Geological Materials & Bio-mineral systems
- Authors
- Dr Elena Suvorova (1)
- Affiliations
-
1. A.V. Shubnikov Institute of Crystallography, Federal Scientific Research Centre “Crystallography and Photonics” of Russian Academy of Sciences
- Keywords
biogenic minerals, bacterial reduction, Mn(II), structure, UO2.
- Abstract text
A direct involving of bacteria in the reduction of metals and metalloids is of considerable interest mainly because of the potential application in the treatment and bioremediation of U contaminated groundwater. The basis of such treatment is the transformation of highly soluble salts of U(VI) to sparingly soluble uranium (IV) oxide. In this work, the particular interest was in identifying and characterizing of the U-containing compound produced by metal-reducing bacteria in the presence of Mn impurities using TEM, electron diffraction, X-ray EDS and EELS. An additional objective is to provide insight methodological aspects, revisiting the TEM data coupling with electron diffraction simulation and structure modelling to propose the mechanism of higher resistance of smaller UO2 particles with adsorbed Mn acetate compared to the larger pure particles.
Biogenic samples were prepared under anoxic conditions at EPFL, Switzerland. A colony of Shewanella oneidensis MR-1 was grown in LB medium. Reduction of U(VI) from uranyl acetate was carried out at pH 6.3 within 12 h. The MnCl2 solution was added to adjust to 0.1, 0.25, 0.5, 1.0, 2.5, 5.0 and 8.0 mM concentrations. The cells and resulting nanoparticles were collected by centrifugation and washed with anoxic H2O. Samples for TEM study were prepared by drying the colloids on a C-coated copper grid or as 50 - 80 nm slices of epoxy with embedded cells and particles. The structure and chemical composition of the particles were studied by conventional TEM, HRTEM, STEM, SAED, X-ray EDS and EELS. The data were processed with the Gatan Digital Micrograph software, INCA (Oxford) and ESPRIT software (Bruker). The simulation and interpretation of SAED patterns and FFTs of HRTEM images and structure modelling were performed with the JEMS software (Stadelmann, 2019).
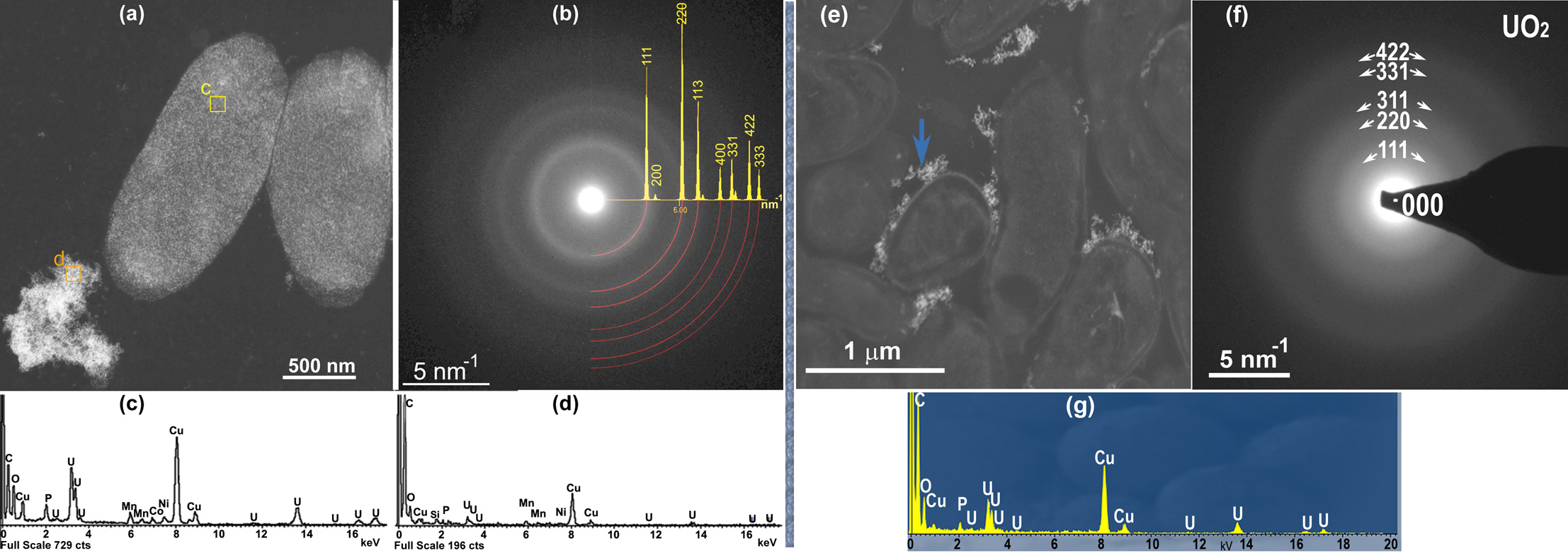
Cells covered with nanoparticles and nanoparticle agglomerations located only in the extracellular space are presented in Fig.1. Phase composition of minerals is derived from the experimental SAED patterns which were compared with the simulated ones for different U oxides (UO3, U3O8, UO, UO2 and U4O9). Analysis showed that the simulated SAED patterns from the UO2 phase with cubic structure (sp.gr. Fm-3m, a=0.544 nm) had the best match with the experimental ones despite the broaden rings due to the size effect (Fig.1).
Figure 1. TEM image of cells covered with UO2-Mn particles and particle agglomerates in an extracellular space (a, e), X-ray EDS spectra showing the particle composition (c, d and g), SAED patterns obtained from particles (b, d) with superimposed JEMS simulated ring pattern (b).
Quantitative data obtained from X-ray ED spectra showed the significant amount of O which cannot be explained by the presence of UO2 only. The balance of elements was maintained in accordance with the stoichiometric formulas to find the composition of UO2 phases mixture and Mn containing phases. The CH3COO2- ions being the part of uranyl acetate and one of the major organic acid metabolites after bacterial reduction of U(VI) was assuming to interact with Mn2+ ions and form hydrated Mn (II) acetates and absorbed on the UO2 nanoparticle surface. The formation of Mn acetate dihydrate or/and tetrahydrate corresponds well to elemental concentrations expressed in atomic % for the samples of assumed thickness ranged from 10 to 60 nm within the experimental error.
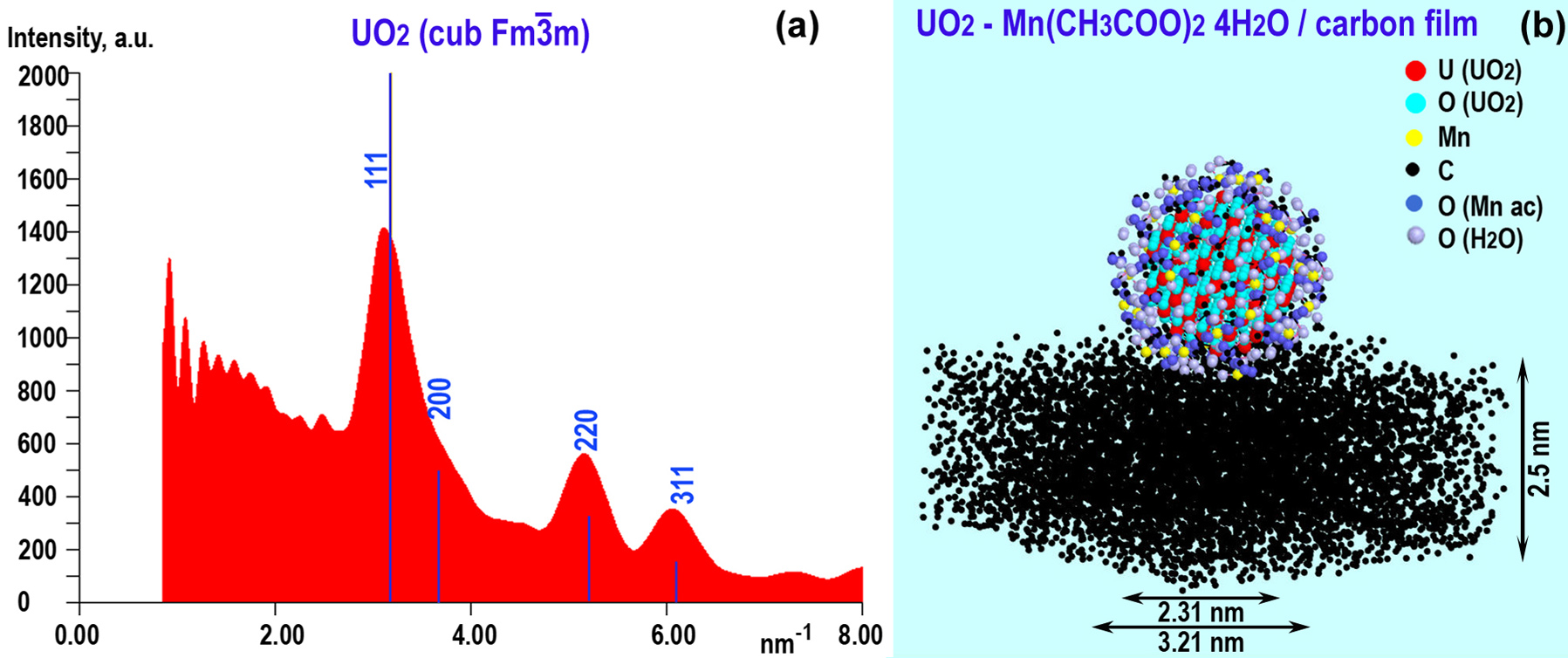
The ab initio simulation of the ring diffraction pattern profile and the structure model of UO2/Mn acetate tetrahydrate core-shell particles on the 2.5-nm carbon film is shown in Fig.2. The presence of Mn acetates in the given concentrations did not affect the positions of the peaks in electron diffraction patterns from UO2 and show the good match with the experimental diffraction patterns.
Figure 2. Simulated intensity profile of the UO2 / Mn(CH3CO2)2 ×4H2O ring diffraction pattern with UO2 reflections (a), structural model of the UO2/ Mn(CH3CO2)2 ×4H2O particle on the C film (b).
EELS was applied to ascertain if mixed chemical states of U (for instance, traces of the remaining U6+). In opposite to some reports that the electron beam tends to change elemental oxidation state of U during EEL spectra acquisitions, we did not observe phase transformations as well the appearance of other U phases in detectable quantities.
The average size of particles was estimated from experimental and simulated electron diffraction patterns while particle size distributions were derived from HR (S)TEM images. It was found that UO2 particle sizes decrease with increase of Mn2+ concentration in solutions. The smaller the size of the particles, the farther their size distribution from the Gaussian shape, approaching the right skewed lognormal distribution.
Modeling the small UO2 particles with non-integer number of cells and the corresponding them the simulated HRTEM images showed that it is possible to visualize particles even smaller than 1 nm using the microscopes without aberration corrections. However only particles of about 1.1 – 1.2 nm in diameter were found what correspond to the model with diameter of 1.16 nm. The particles smaller than this size (0.76 nm) with 4 U atoms surrounded by 16 O atoms are hardly believable to stay independently.
Adsorption of Mn acetates on the surface of UO2 nanoparticles and formation of the shell can explain the increase of suppression of particle growth and the increase in particle stability and dissolution resistance. The process of biogenic removal of U6+ from contaminated water can be efficient also for the simultaneous removal of Mn2+ ions.
- References