Caught in transition: Nanoscale analysis and molecular level characterisation of collagen mineralisation by complementary use of electron microscopy and in situ Raman microspectroscopy
- Abstract number
- 314
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.314
- Corresponding Email
- [email protected]
- Session
- PSA.6 - Geological Materials & Bio-mineral systems
- Authors
- Emma Tong (4), Dr Brian Wingender (3), Professor Roland Kroger (4), Professor Laurie Gower (2), Dr Julia Parker (1)
- Affiliations
-
1. Diamond Light Source
2. University of Florida
3. University of North Florida
4. University of York
- Keywords
collagen type 1, electron microscopy, mineralisation, Raman
- Abstract text
Bone is a fascinating and critically important biocomposite combining hardness and toughness through its hierarchical organisation facilitated by the nano-level organisation of an inorganic mineral [1], hydroxyapatite (HAp), and a protein, collagen type I, along with a minor fraction of non-collagenous proteins (NCP’s) and polysaccharides that affect the bone formation process largely due to their calcium binding potential. Despite intensive research the mechanism behind the mineralisation of the collagen matrix, constituted of fibrils with approximately 100 nm diameter, is still a central problem in the field of bone research. Current understanding is that the mineral formation occurs via an amorphous precursor phase that infiltrates the collagen fibrils and subsequently crystallises [2, 3] However, the fundamental details of collagen infiltration and crystal growth remain controversial. A key problem is the understanding of the infiltration and mineralisation dynamics which we addressed by using ex situ transmission electron microscopy (TEM) in conjunction with electron diffraction for high-resolution analysis of the mineral phase as well as scanning electron microscopy (SEM) complemented by novel in situ Raman microspectroscopy providing insights into the molecular level transport and transformation processes of the phosphate precursor phases. This approach allowed us to study the time dependence of precursor transport, intermediate phase formation and subsequent mineral growth using a model system of collagen mineralisation, namely the polymer induced liquid precursor (PILP) method. Our studies show that both intrafibrillar and extrafibrillar mineralisation occurs starting from an amorphous calcium phosphate (ACP) precursor via most likely octacalcium phosphate (OCP) to HAp in a time frame of approximately two hours whereas the complete mineralisation of collagen happened over a period of nine hours. Hence a rapid mineral phase transformation precedes the subsequent slower mineral growth process.
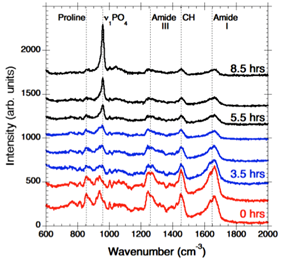
In order to identify the molecular level processes involved in the precursor transport and phase transformation, we have used an in vitro model system, the PILP process, by which collagen is mineralised in both intra- and extra-fibrillar spaces [2]. This leads to a nanostructural organisation that emulates key aspects of bone formation. Our in vitro model system employs a process-directing polymer as a biogenic analogue to the NCP’s found in native bone formation. Using a bespoke in situ heated liquid cell to maintain physiological temperatures within a Raman spectrometer, we have examined collagen mineralisation in real time, and observed peak development indicative of the transition from an amorphous precursor phase, ACP/OCP through to the formation of hydroxyapatite (HAp) crystals (Fig. 1).
Fig.1 Time resolved Raman spectra showing PO4 peak shift and evolution.
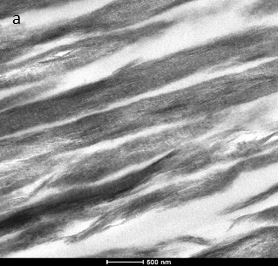
A detailed analysis of the resulting mineralised collagen was performed by TEM. Bright field TEM (BFTEM) images showed densely packed, aligned collagen fibrils. The characteristic D-banding of the collagen fibrils (indicative of the periodic arrangement of gap- and overlap regions within the collagen fibers) can be clearly observed (Fig. 2).
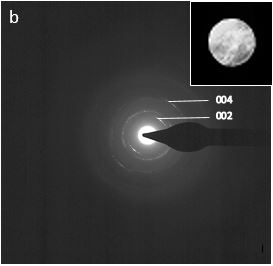
Fig. 2 (a) BFTEM image of mineralised collagen fibers; (b) Selected area diffraction pattern of mineralised collagen showing the (002) and (004) reflection arc.
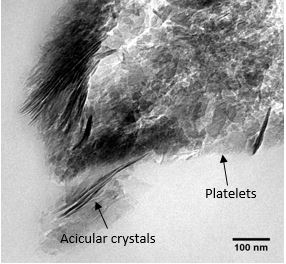
Selected area electron diffraction (SAED) patterns and high resolution TEM (HRTEM) images are consistent with those for hydroxyapatite crystals. The (002) and the (004) reflection arcs can be observed in the diffraction patterns indicating that the [001] c-axis of the HAp crystals are roughly aligned with the collagen fibril axis within an angular range of approx. ±20˚ (Fig. 2 (b)). Furthermore, the BFTEM images revealed the coexistence of two crystal morphologies, platelet and needle like in shape (Fig. 3).
Fig. 3 BFTEM image of a mineralised collagen fiber showing the coexistence of two crystal morphologies.
Our findings indicate that we can observe both intrafibrillar and extrafibrillar mineralisation akin to that observed in native bone. This work further allows for the quantitative characterisation of the kinetics of precursor infiltration and crystallisation, which are strongly dependent on the choice of polymer used for calcium and phosphate transport and opens up new avenues for a full understanding of bone mineralisation.
- References
1. Reznikov, N., Bilton, M., Lari, L., Stevens, MM., Kröger, R. (2018). Fractal-like hierarchical organization of bone begins at the nanoscale. Science, 360.
2. Gower, LB. (2008). Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chemical Reviews, 108(11), 4551-4627.
3. Olszta, M., Cheng, XG., Jee, SS., Kumar, R., Kim, YY., Kaufman, MJ., Douglas, EP., Gower, LB. (2007). Bone structure and Formation: A new perspective. Materials Science and Engineering. 58(3-5), 77-116. https://doi.org/10.1016/j.mser.2007.05.001.