Coarsening of nanoparticles during catalysis: is ostwald ripening the culprit?
- Abstract number
- 524
- Event
- Virtual Early Career European Microscopy Congress 2020
- Presentation Form
- Submitted Oral
- DOI
- 10.22443/rms.emc2020.524
- Corresponding Email
- [email protected]
- Session
- PST.6 - In-situ and in-operando microscopy
- Authors
- Dr Rhys Lodge (2), Dr Graham Rance (2, 1), Dr Michael Fay (1), Professor Andrei Khlobystov (2, 1)
- Affiliations
-
1. Nanoscale and Microscale Research Centre, University of Nottingham
2. School of Chemistry, University of Nottingham
- Keywords
IL-TEM, Nanoparticles, Carbon Nanotubes, Palladium, Suzuki-Miyaura
- Abstract text
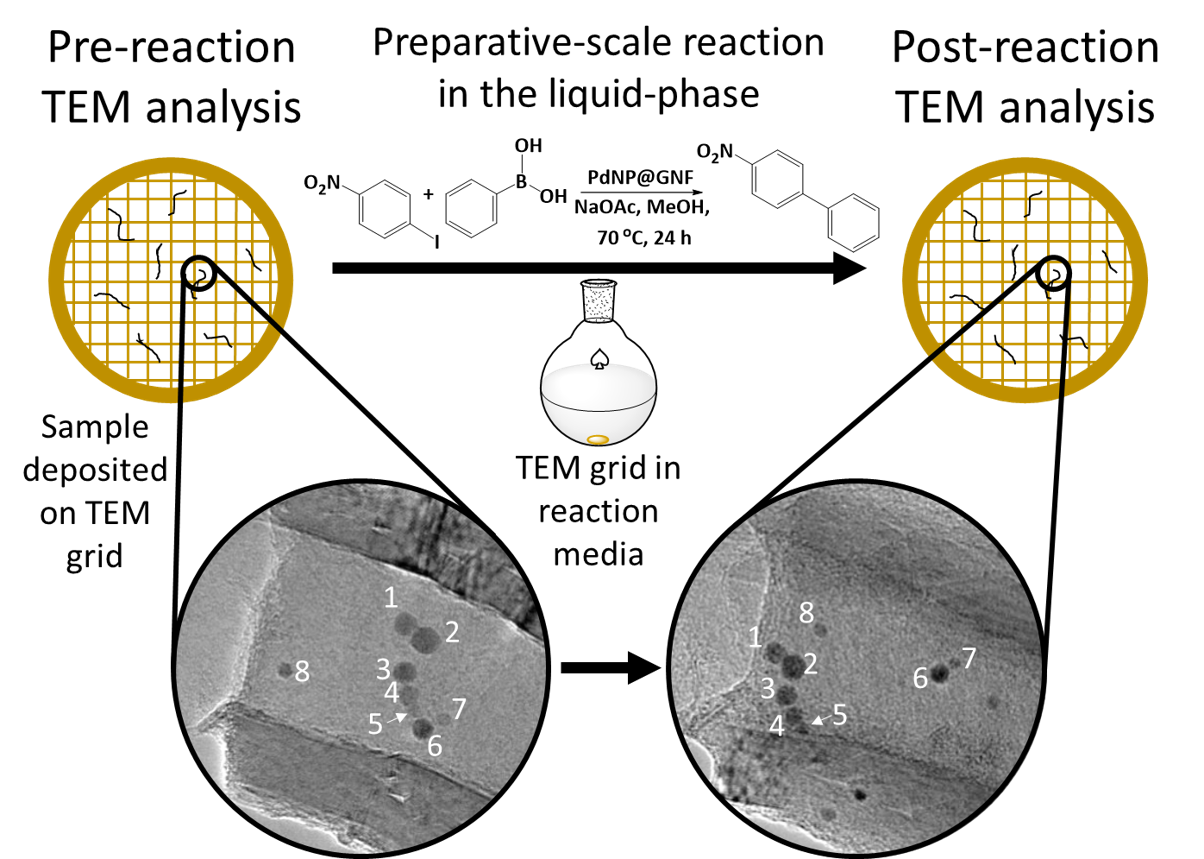
Summary: The evolution of individual palladium nanoparticle (PdNP) catalysts in hollow graphitised nanofibres (GNF) in the liquid-phase Suzuki–Miyaura (SM) reaction has been appraised by identical location transmission electron microscopy (IL-TEM) [1].
Introduction: The highly dynamic, metastable nature of metal nanoparticles (NP) often leads to increasing NP sizes during catalytic cycles, which decreases the number of active surface atoms and reduces catalytic activity [2-4]. Consequently, there has been a significant drive to better understand the fundamental mechanisms that lead to nanoparticle coarsening and deactivation. Although a multitude of microscopy techniques have previously been used to appraise NP growth, one of the most widely utilised is TEM, with two specific imaging strategies, namely the sampling and in-situ environmental TEM (ETEM) methods, commonly employed [5-8]. These two techniques have provided a wealth of information on NP growth mechanisms; however, understanding these processes over long durations under catalytic liquid-phase conditions, and their effect on individual NP, is incredibly difficult or impossible by sampling and ETEM techniques. This led to the development of IL-TEM – pioneered by Mayrhofer et al. in 2008 [9]. The IL-TEM process involves deposition of the sample onto a finder grid, areas are then analysed, before the grid and catalyst are exposed to the reaction conditions (figure 1). The same areas are then relocated in the TEM using the alphanumerical notation and re-analysed allowing for changes to individual nanoparticles to be elucidated. This technique has been primarily applied to studying electrochemical reactions but the capacity for this technique to study catalytic NP in bulk, liquid-phase reaction conditions warranted investigation.
Method/materials: Herein, we deposited PdNP confined within GNF, a unique hybrid carbon nanotube, onto a gold mesh-graphene oxide/lacey carbon film finder grid and studied the effect of a SM cross-coupling reaction on the PdNP over two cycles using IL-TEM.
Results/discussion: Several PdNP contained within GNF were identified prior to the application of the grid into the SM reaction. The grid was then deposited into the reaction mixture and the PdNP were relocated and re-examined by TEM following each of the two cycles. The subsequent image analysis highlighted two attributes of the PdNP: i) regions containing larger PdNP adjacent to smaller PdNP did not lead to the formation of one large NP, as would be expected in the commonly accepted Ostwald ripening mechanism, rather the smaller NP began fusing together implying that the mechanism was based on particle migration and coalescence; and ii) there was a size dependent migration of NP within the GNF, nanoparticles that were commensurate in size with the step-edge structure of the GNF (<5 nm) were only observed to undergo transverse migration around the internal circumference of the GNF, whereas particles larger than the step-edge (>5 nm), which consequently had a smaller van der Waals interaction with the step-edge, were able to undergo a unidirectional, longitudinal migration down the growth axis of the GNF. EDX spectroscopy detected the presence of reagents in the GNF and 1H NMR spectroscopy of the reaction mixture confirmed the catalytic nature of the PdNP with a calculated turnover frequency of 42,000 h-1.
Conclusion: We have successfully applied a combination of IL-TEM analysis and the nano test tube approach to investigate the behaviour of supported metal nanoparticles as catalysts of liquid-phase, preparative reactions. We have demonstrated that IL-TEM analysis of PdNP within GNF allows the evolution of individually identifiable nanoparticles to be tracked, demonstrating that PdNP remain solid during the reaction (i.e. do not dissolve and re-precipitate as commonly perceived), and that nanoparticle migration and coalescence is the principle mechanism decreasing the active surface area under the conditions of the Suzuki–Miyaura reaction. These results suggest that the pseudo-homogeneous mechanism proposed for palladium and other metal nanoparticles, through dissolution-precipitation events, may not be as prevalent as expected from previous studies using the sampling approach, thus highlighting the importance of IL-TEM for providing key mechanistic insights for nanocatalysis. Information gained from our observations on the relationship between the growth and migration of nanoparticles and the morphology of the graphitic surface offers a powerful strategy for engineering highly durable carbon supports, with step-edges matching the size of catalytic centres. This may ultimately lead to the inhibition of nanoparticle growth and subsequent deactivation as coarsening by particle migration and coalescence can effectively be controlled in such a fashion. As such, we offer new hope for the development of future nanocatalysts, particularly those applicable to environmentally and economically sustainable chemical synthesis.
Figure 1: Schematic of IL-TEM as a tool to study the evolution of PdNP following exposure to the bulk, liquid-phase conditions of a Suzuki-Miyaura reaction
- References
[1] RW Lodge et al, Nanoscale 10 (2018), p. 19046.
[2] SG Rinaldo et al, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys. 86 (2012), p. 041601.
[3] L Shang et al, Angew. Chem. Int. Ed. 53 (2014), p. 250.
[4] H-H Zhang et al, Tetrahedron 70 (2014), p. 6188.
[5] B Cornelio et al, J. Mat Chem A 3 (2015), p. 3918
[6] GA Rance et al, Chem. Commun. 49 (2013), p. 1067.
[7] SB Vendelbo et al, Nat. Mater. 13 (2014), p. 884.
[8] J Yang et al, J. Am. Chem. Soc. 141 (2019), p. 763
[9] KJJ Mayrhofer et al, J. Power Sources 185 (2008), p. 734.