Intraparticle heterogeneity of composition and structure in Ca–Mg carbonate minerals precipitating from a shallow, alkaline lake
- Abstract number
- 956
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.956
- Corresponding Email
- [email protected]
- Session
- PSA.6 - Geological Materials & Bio-mineral systems
- Authors
- Mihály Pósfai (2), Péter Pekker (2), Zsombor Molnár (2), István Dódony (2), Silvia Frisia (1), Patrick Meister (3)
- Affiliations
-
1. University of Newcastle
2. University of Pannonia, Nanolab
3. University of Vienna
- Keywords
calcite, dolomite, ordering, precipitation, lake
- Abstract text
The precipitation of carbonate minerals from surface waters has important implications for Earth’s climate, the formation of carbonate rocks, and aquatic ecology. An enigmatic problem of carbonate mineralogy is the formation of dolomite, CaMg(CO3)2 with an ordered arrangement of alternating layers of Ca and Mg in its structure, under Earth surface conditions; various mechanisms, including diagenetic transformation of magnesian calcite and the catalytic effect of microorganisms and their extrudates have been suggested [1]. Here, we present results on the microstructures of Ca–Mg carbonates that precipitated from a shallow lake, and show the presence of nm-scale domains with differently ordered arrangements of Ca and Mg.
We studied particles (< 2 μm fraction) collected from the sediment of Lake Neusiedl, a large but extremely shallow (< 1.8 m deep), alkaline (pH > 8.5) lake with a high dissolved Mg/Ca ratio (> 7), situated in Central Europe, at the border of Austria and Hungary [2]. In order to study structural and compositional variations of carbonates on the nm scale by using a Thermo Scientific Talos F200X G2 scanning transmission electron microscope, both in conventional transmission (TEM) and scanning transmission (STEM) modes. High-angle annular dark-field (HAADF) STEM images were obtained, along with selected-area electron diffraction (SAED) patterns, and elemental maps were acquired using energy-dispersive X-ray spectroscopy (EDS) in STEM mode.
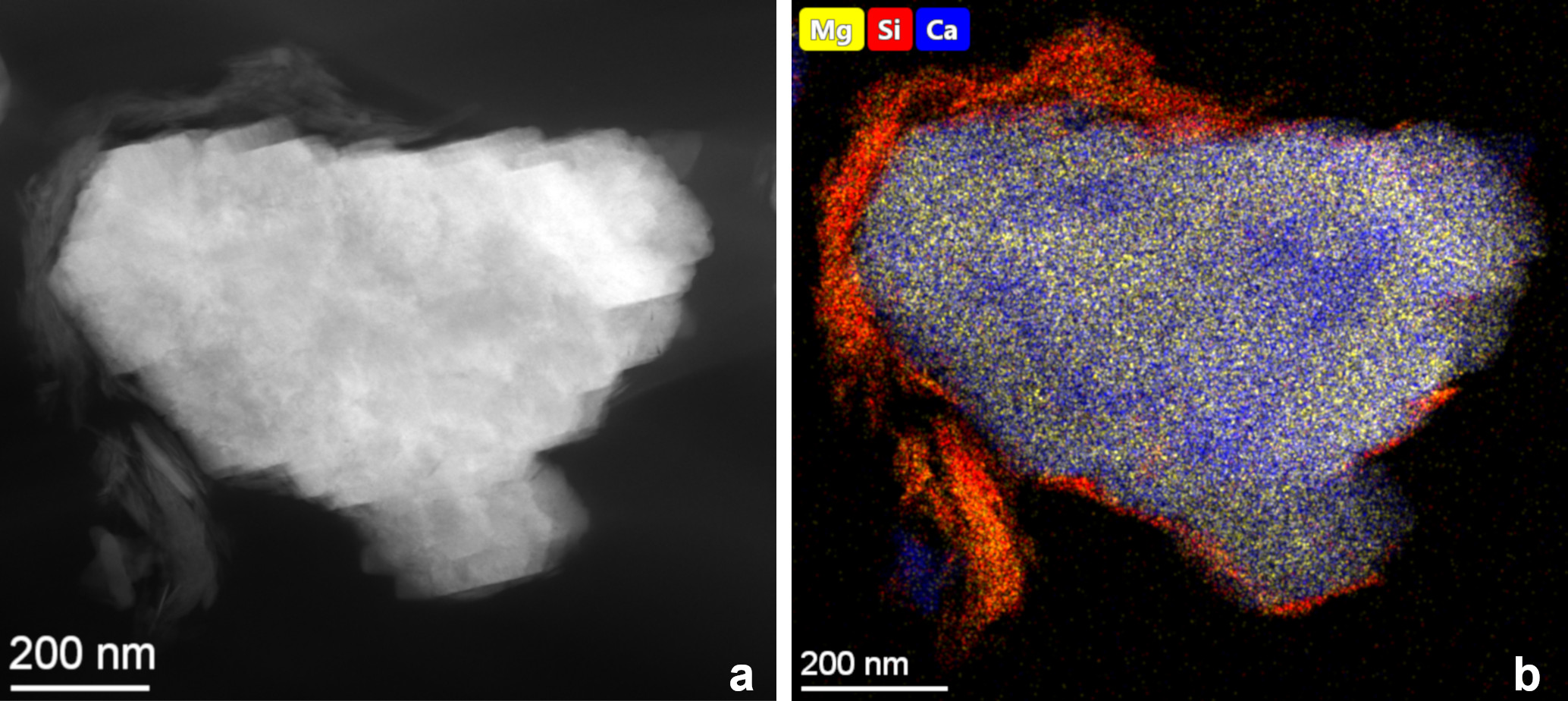
Carbonate mineral particles appear as aggregates of many individual nanocrystals but are in fact single crystals (Fig. 1a). Typically, each carbonate particle is partly rimmed by thin layers of Mg-bearing smectite (a clay mineral), as illustrated by the distribution of Si (indicated in red color) around the carbonate particle in Figure 1b. Similar associations of smectite with carbonates were observed in another large lake and interpreted as formed by the heterogeneous nucleation of carbonate on pre-existing flakes of smectite [3,4]. Elemental maps show a wide range of Mg/Ca ratios, both between and inside individual particles: an analysis of 400 particles produced an essentially continuous distribution from almost stoichiometric calcite to a slightly Ca-rich dolomite composition. A striking feature of some particles is intraparticle compositional heterogeneity: distinct changes in the Mg/Ca ratio can be observed, typically (but not exclusively) in concentric zones (Fig. 1b). In such particles the Mg-rich zones have dolomite-like compositions (Mg/Ca atomic ratio close to 1), whereas the rest of the particle displays a variety of Mg/Ca atomic ratios up to about 0.5. These compositional variations are reflected in structural changes as well: whereas SAED patterns obtained from the less Mg-rich regions show no ordering reflections, patterns from the Mg-rich regions typically display ordering reflections (such as the 003) typical of the dolomite structure.
Figure 1. (a) HAADF image and (b) STEM-EDS elemental map of a typical carbonate particle from the sediment of Lake Neusiedl. As indicated by the disribution of Si, the particle is rimmed by a thin veil of the clay mineral smectite. Intraparticle compositional heterogeneity is visible by the variation of predominantly yellow and blue zones that correspond to Mg-bearing calcitic and dolomitic compositions, respectively.
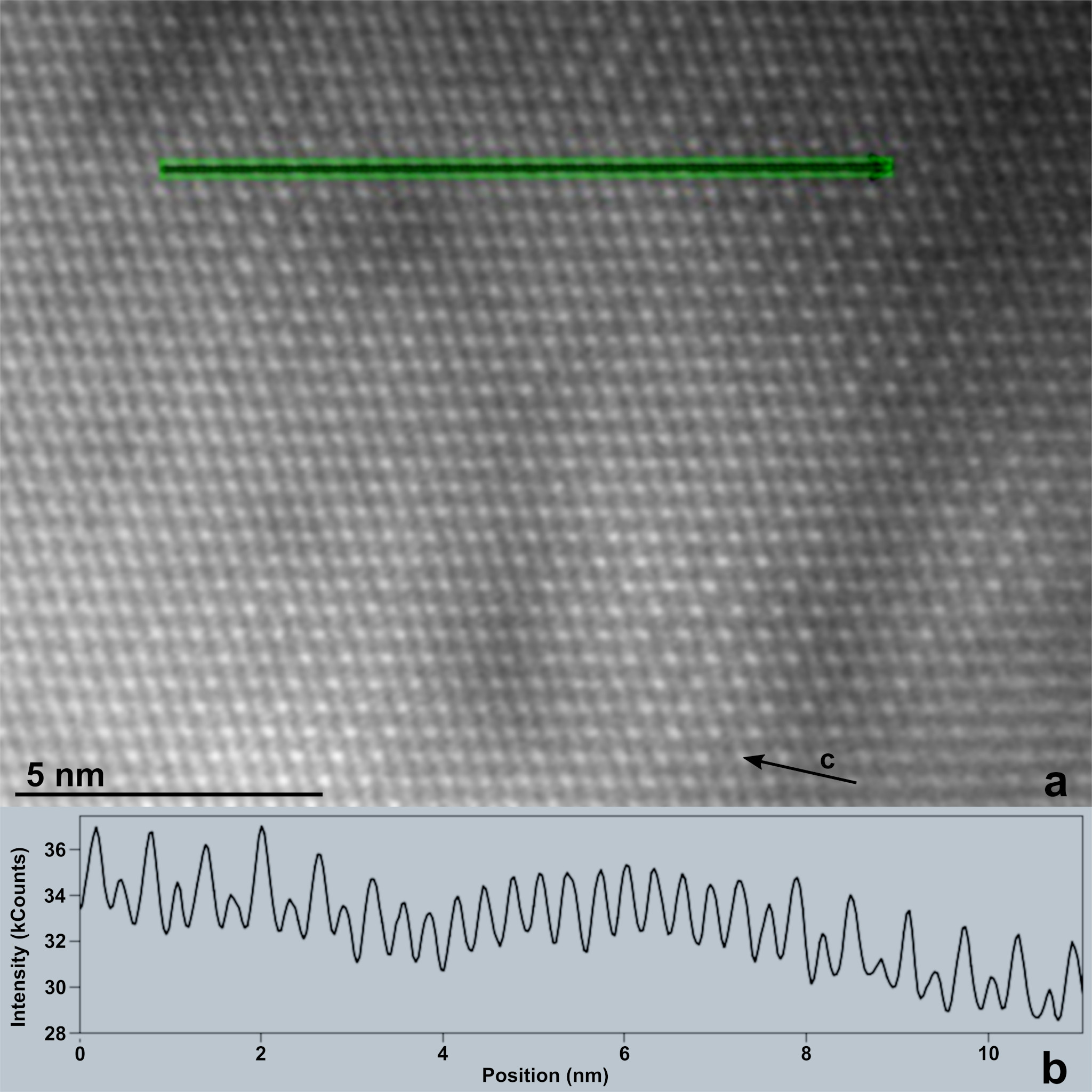
High-resolution TEM and HAADF images reveal an additional structural complexity within the Mg-rich zones, suggesting that the degree of Mg/Ca ordering varies on the nm scale. Figure 2a shows a high-resolution HAADF image (obtained with the electron beam parallel to the calcite [100] direction), in which individual cation columns are resolved as dots with bright contrast. Since the intensity of each dot is directly related to the square of the total atomic number (Z2) within the column, an ordered alternation of Ca and Mg columns will produce dots with strong and faint contrast, respectively, whereas a disordered arrangement of Ca and Mg will produce dots with equal contrast. As seen from the example shown in Figure 2b, nm-scale domains (less than 5 nm in diameter) with ordered arrangements of Ca and Mg ions alternate with disordered (or less ordered) domains of similar sizes. Thus, our results suggest that nm-scale ordered dolomite forms in the lake.
Figure 2. (a) High-resolution HAADF image obtained from a region of a carbonate particle with dolomitic composition, indicating nm-scale domains of ordered and disordered arrangements of Ca and Mg. (b) Intensity profile of image contrast along the line shown in (a), suggesting the presence of alternating Ca and Mg atomic columns on the two sides, and no ordering (atomic columns with approximately equal numbers of Ca and Mg atoms) in the middle of the section.
Our observations of intraparticle compositional heterogeneity, i.e., the alternation of ‘calcite’ and ‘dolomite’ zones, can be interpreted as evidence for fluctuations in lakewater chemistry. As was shown earlier [2], in Lake Neusiedl the pH can vary between 7.5 and 9. Metastable Mg-bearing calcite partially dissolves under lower pH, and the formation of thermodynamically stable dolomite becomes possible. In contrast, at higher pH metastable Mg-bearing calcite forms because of a kinetic barrier to the precipitation of stable, ordered dolomite. The observation of nm-scale structural heterogeneity (variations in Mg/Ca ordering) is a novelty for a contemporary carbonate system and was only observed in older rocks [5]. Further analysis of HAADF images is in progress, in order to be able to put forward a feasible explanation for the formation of nm-scale structural fluctuations of cation ordering.
- References
[1] JM Gregg et al, Sedimentology 62 (2015), 1749.
[2] D Fussmann et al, Biogeosciences Discussions (2019), https://doi.org/10.5194/bg-2019-449.
[3] I Nyirő-Kósa et al, Earth and Planetary Science Letters 496 (2018), 20.
[4] M Fodor et al, Chemical Geology 538 (2020), 119497.
[5] Y Fang and H Xu, Journal of Sedimentary Research 89 (2019), 537.
[6] This research was supported by NKFIH grants no. K116732, GINOP-2.3.2-15-2016-00017and GINOP-2.3.3-15-2016-0009.