LIVE DYNAMIC IMAGING OF NANO SCALE POSTSYNAPTIC STRUCTURES
- Abstract number
- 1196
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.1196
- Corresponding Email
- [email protected]
- Session
- LSA.3 - Applications for imaging sub-cellular events at high resolution
- Authors
- Dr. Eduard Korkotian (1, 2), Lilia Kushnireva (2)
- Affiliations
-
1. Department of Neurobiology, The Weizmann Institute
2. Department of Zoology, Perm State University
- Keywords
calcium store, dendritic spine, spine apparatus, synaptic plasticity
- Abstract text
Our study describes local calcium dynamics in memory-type dendritic spines, which contain independent nano-scale calcium stores. We show for the first time that activity-derived memory spines initiate highly restricted calcium waves, based on store-operated calcium entry (SOCE) process. Our findings provides a new mechanism of local synaptic plasticity.
Dendritic spines – the small (about 1 µm - long) postsynaptic structures of neuronal cells remain poorly understood in the context of their functions, synaptic plasticity and local memory mechanisms. Some large spines contain a unique nano-structure called spine apparatus (SA). Following the past ten years of intensive research, SA of those large and highly channel-enriched “memory” spines has been associated with completely functional calcium storage, which is rather independent of entire endoplasmic reticulum (ER) network lying in parent dendrites. Among others, this finding raised the question of local store refiling process and its possible involvement in spine plasticity. Our current study addressed some components of this complex issue.
We carried out our study in rat primary hippocampal cultured neurons, transfected with the following plasmids: BFP as cell morphology marker, DsRed-synaptopodin as SP marker and STIM-cherry as a component of SOCE-related STIM/Orai complex. In some experiments, we used RCaMP or GCaMP calcium sensor proteins associated with ER, detecting calcium levels inside the internal stores. Additionally we pre-incubated cultures with high affinity cytosolic calcium sensor Fluo-2(AM) for 1 hour prior recording. In order to deprive spontaneous activity we used a blocker mix, containing APV, DNQX and TTX in order to block NMDA, AMPA and voltage-gated sodium channels, respectively. We achieved the efficient depletion of internal stores by incubation of cultures in calcium-free extracellular medium during 20 minutes. For rapid and high-resolution multichannel imaging, we used Zeiss 880 confocal microscope, equipped with appropriate lasers. For caffeine-dependent calcium release from rynanodine sensitive stores, we used its bath of pipette puff application.
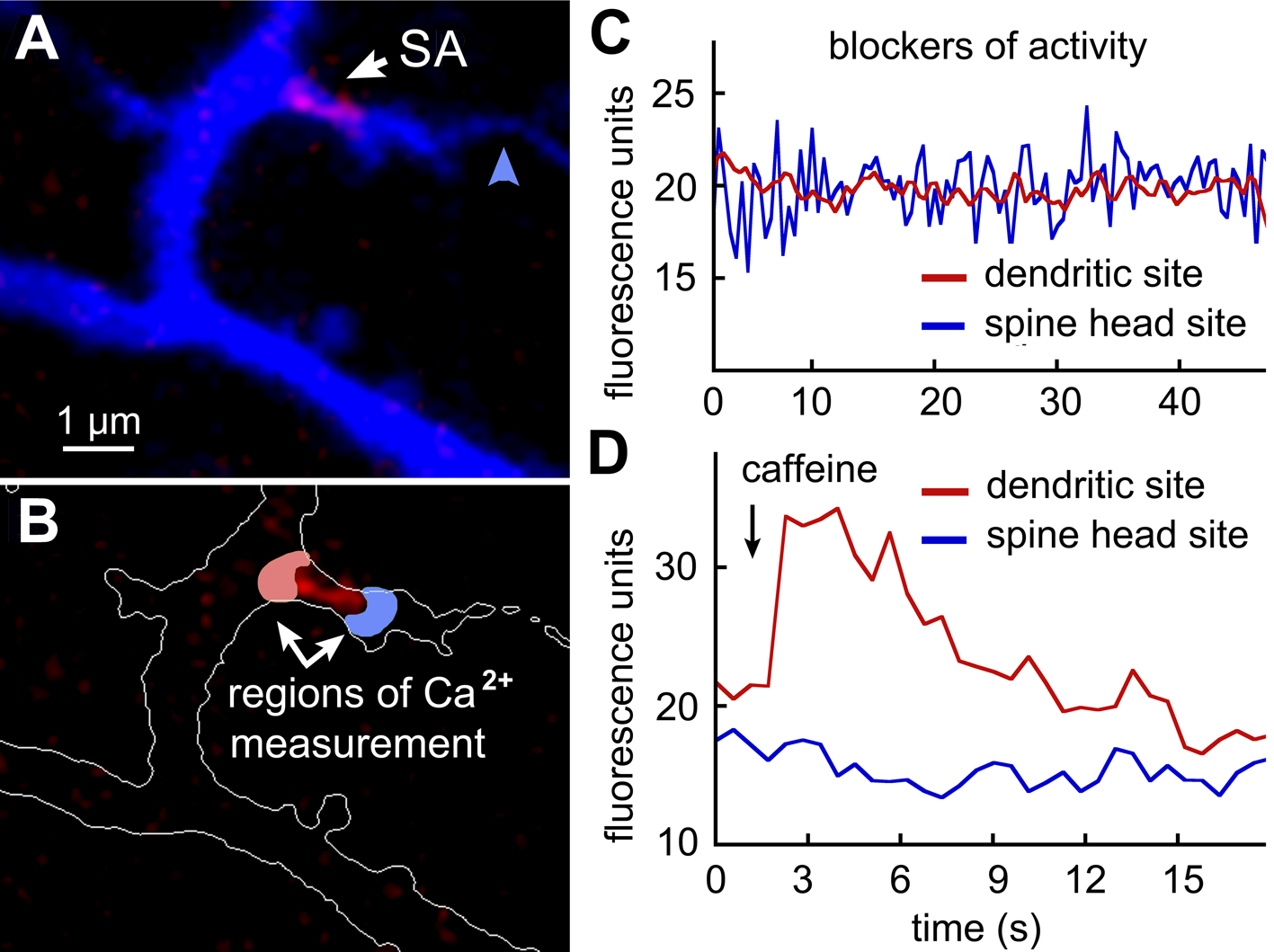
A subset of large dendritic spines, receiving axon inputs (blue arrowhead on panel A of supplementary figure) were containing SA (white arrow). In recent study [1] we could show a unique uneven distribution of SERCA pumps and ryanodine calcium-releasing receptors (RyR) on SA. Particularly, SERCA pumps accumulated on the spine head site of SA (blue region on panel B), while RyRs – on the dendritic site (red region on panel B). We first incubated cells in calcium-free extracellular medium during 20 minutes. This could deplete SA-associated stores which was detected by caffeine application (5 mM), (n=7, p<0.0001). We then treated cultures with activity blockers (APV, 30 µM; DNQX, 15 µM and TTX, 1 µM) in order to prevent the recovery of spontaneous activity after switching to a solution containing the regular concentration of extracellular calcium (2 mM). In the following recording from the SA-adjacent region facing to the spine head, we could observe relatively slow (0.53±0.02 Hz) and low amplitude (2.28±0.14 arbitrary units) irregular calcium waves, associated with SOCE (panel C, blue trace). Such waves were nearly never observed in the SA-adjacent region, facing towards the parent dendrite (0.09±0.01 Hz) (panel C, red trace) or in SA-negative spine heads (0.18±0.04 Hz) (ANOVA, p<0.001).
Figure 1. Store-operated calcium entry (SOCE) into dendritic spine, containing spine apparatus.
A - Fragment of BFP-labeled cultures rat hippocampal neuron, containing dendritic spine with axon terminal (blue arrow) and spine apparatus (SA, red label). B – Two regions of interest (ROI) adjacent to SA and facing towards the spine head (blue) and dendritic shaft (red). C – Calcium waves in the blue ROI recorded following depletion of SA-store and in the presence of activity blockers (see text for details). D – Caffeine-dependent calcium transient released from SA through ryanodine receptors and recorded in the red ROI.
We then tested the level of replenishment of SA using caffeine bath of puff application in the presence of the same activity blockers. In this case, we could observe a clear cytosolic calcium transient, associated with its release from SA storage and directed towards the parent dendrite; while spine head SA-adjacent areas were either fully or partly absent of calcium transients (panel D, p<0.005).
In different series of experiments, we aimed to detect calcium levels directly inside SA, using ER-associated genetically encoded calcium sensor. We used the same experimental paradigm to observe calcium fluctuations directly inside SA of memory spines.
In order to confirm the obtained data, we attempted to trace the movement of the STIM protein aggregate (puncta) under the following three conditions: (1) before elimination of extracellular calcium from the solution, during the washout of calcium (2) and, finally, after the returning to initial calcium-containing medium (3). Our data indicate that under normal conditions (1), STIM points are mainly concentrated in the dendritic shaft. They are usually absent in both SA-positive and SA-negative spines. However, after the depletion of local calcium storage, puncta begin to move in the direction of SA-positive spines penetrating into them (2). Finally, after the return to normal conditions, a certain dynamic separation of STIM aggregates from spines is observed.
Taken together, our data indicate the existence of a new and previously undescribed mechanism of local, selective replenishment of isolated calcium depots in memory spines through SOCE. This mechanism may play a role in the absence of intercellular interaction, which typically restores calcium under the normal conditions. It can be particularly important for the selective enhancement of memory spines and the implementation of synaptic plasticity mechanisms, which depend on the local calcium release. Finally, this mechanism can be very useful in neurodegeneration, such as Alzheimer's disease, when some spines lose their natural afferent inputs.
Conclusions:
1. There is a selective mechanism for replenishment of calcium stores in those spines that contain SA.
2. Mechanism is associated with local and dynamic functioning of the SOCE system.
3. The replenishment of calcium stores is carried out from the head side of dendritic spines.
4. On the contrary, the release of calcium predominantly occurs from the side of SA, directed towards the dendrite.
5. This mechanism is implemented due to the relocation of STIM aggregates from parent dendrites into spines.
- References
[1] Basnayake et al, PLOS Biology, 17 (2019)