One–pot synthesis of core/shell Cu/FePt nanocatalysts for efficient oxygen reduction reaction: morphology and structure evolutions
- Abstract number
- 591
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.591
- Corresponding Email
- [email protected]
- Session
- PSA.5 - Nanoparticles & Catalysts
- Authors
- Dr. Xu Chen (2), Dr. Yi Wang (2), Mr. Kersten Hahn (2), Prof. Dr. Hao Wang (1), Prof. Dr. Peter A. van Aken (2)
- Affiliations
-
1. Hubei University
2. Max Planck Institute for Solid State Research
- Keywords
Electrocatalysis, FePtCu, Intermetallics, Oxygen Reduction Reaction, Transmission Electron Microscopy
- Abstract text
Although fuel-cell vehicles have already been commercially available, the reduction of Pt catalyst in the cathode is highly desirable to lower their costs for massive production.[1,2] Low–Pt intermetallic catalysts have been proven a promising candidate due to their enhanced mass activity and thus reduced Pt content as compared to commercial Pt catalysts, as well as due to the improvement of durability in acidic and oxidative environments.[3] In a system involving Pt and Fe, the Pt−Pt and Pt−Fe bond lengths shorten, when disordered FePt (face-centered cubic) nanocrystals convert into intermetallic FePt (face-centered tetragonal) nanocrystals, which in turn lowers the oxygen-absorption energy and enhances the catalytic activity.[4] While thermal treatment of a dry sample is usually needed for this phase conversion, which causes an aggregation and coalescence of the nanoparticles, a remarkable degradation of nanoparticles’ uniformity and catalysis performance is observed.[5] In contrast, wet-chemistry synthesis stands out as a foremost route to prepare intermetallic nanocrystals without high-vacuum and high-temperature conditions as is the case for thermal annealing.
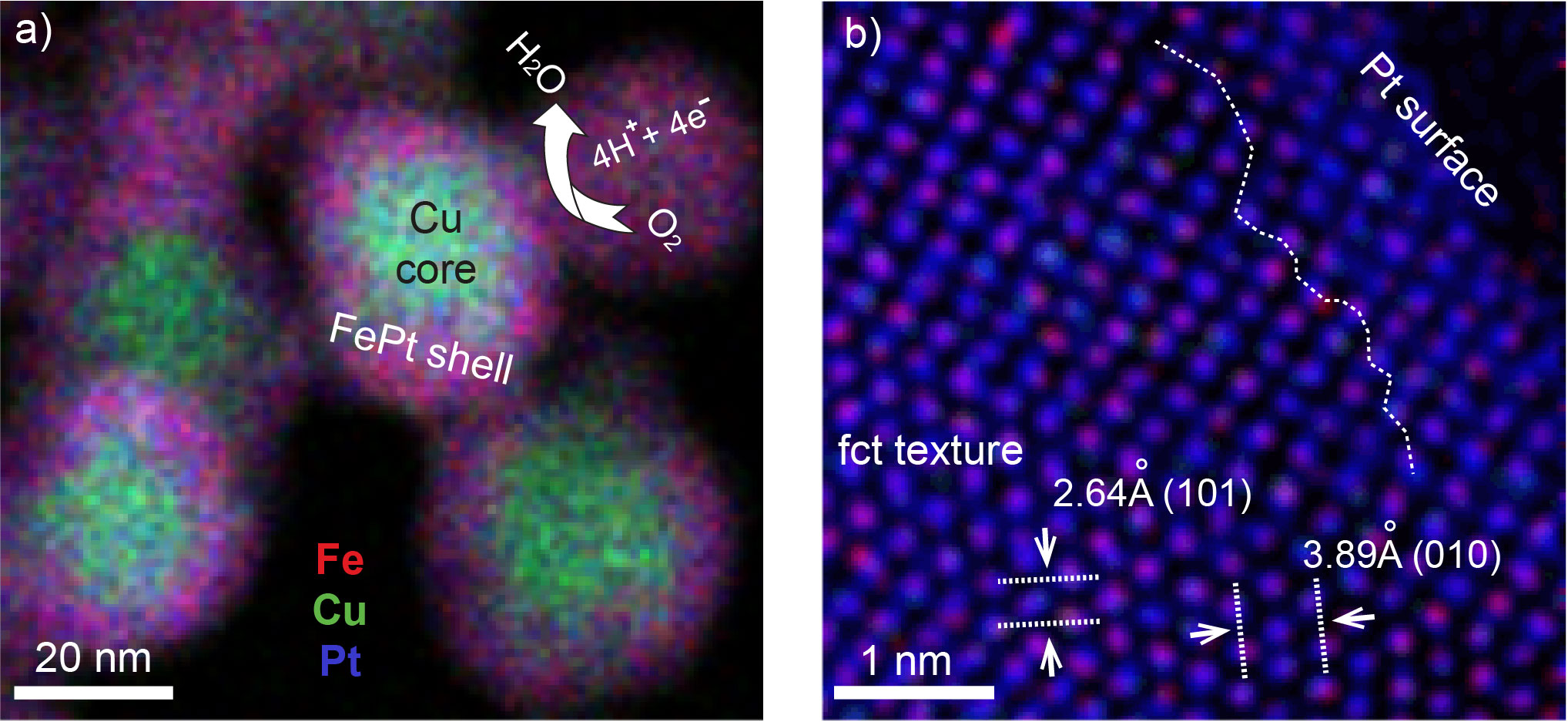
Figure 1. a) STEM–EELS mapping of core–shell Cu@FePt nanoparticles (red–Fe, green–Cu, blue–Pt. b) Atomic–resolved STEM–EELS mapping of the particle surface.[6]
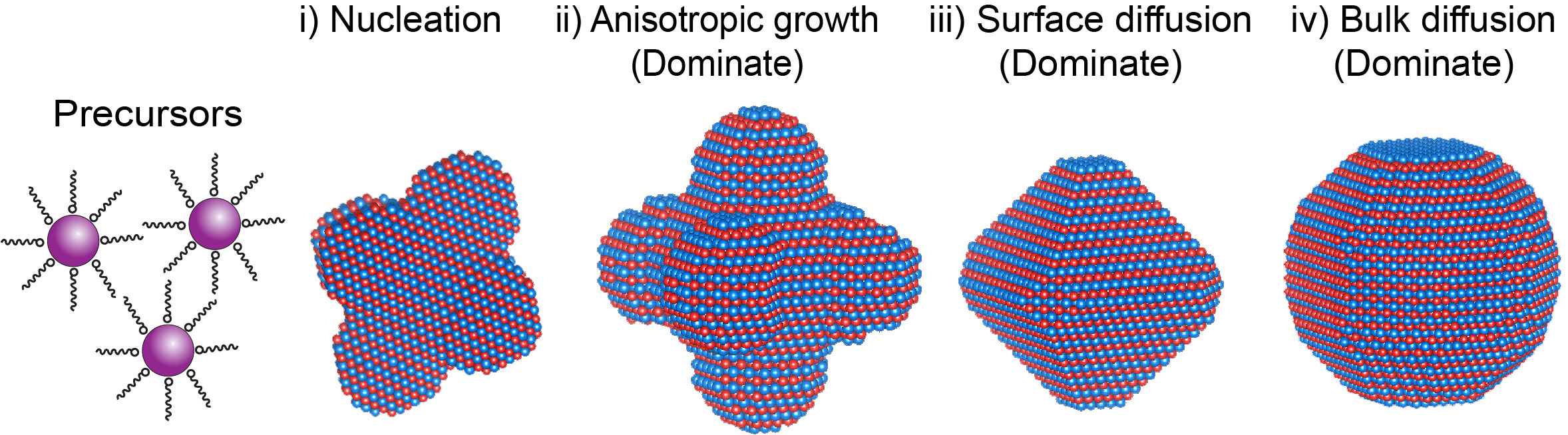
Here, we report a one–pot synthesis of core–shell Cu@FePt nanoparticles with an intermetallic structure, as shown in Figure 1.[6] The core–shell FePtCu catalyst with a Pt-enriched surface exhibits 0.5 A/mgPt mass activity, which is a factor of 4 times higher than that of commercial Pt/C (0.13 A/mgPt). In addition, the current density of the catalyst drops only by 3.0% after 1000 cycles, which is much reduced and better than for Pt/C with a decay of 34.2%. Furthermore, the FePtCu growth and structural evolution in solution have been investigated ex-situ by atomically resolved scanning transmission electron microscopy and electron energy-loss spectrum-imaging techniques. The FePtCu nuclei undergo an element-specific anisotropic growth, more specifically, (i) initially starting from a faceted polyhedron nuclei, to (ii) an anisotropic grow along <100> directions resulting in dendrite-like shapes, to (iii) then evolving into truncated octahedra, and (iv) finally the core–shell Cu@FePt structures turn into a solid-solution alloy, as shown in Figure 2.
Figure 2. Illustration of the morphology evolution of FePtCu nanoparticles during one–pot synthesis at different reaction stages.
These results demonstrate the diversity of the morphology of Pt–alloy nanoparticles via one–pot synthesis, which will aid rational synthesis of low−Pt intermetallic catalysts. Our work also provided a proof of a concept, that the high-performance FePtCu catalyst with not only intermetallic but also with a core–shell structure is attainable.[7]
- References
[1] C. Chen, Y. Kang,..., V.R. Stamenkovic, Science 343 (2014), 1339−1343.
[2] S. Ott, A. Orfanidi,..., P. Strasser, Nature Mater. 19 (2020), 77−85.
[3] J. Li, S. Sharma,..., S. Sun, Joule 3 (2019), 1−12.
[4] D. Y. Chung, S. W. Jun, G. Yoon, et al, J. Am. Chem. Soc. 137 (2015), 15478−15485.
[5] X. Chen, H. Wang, H. Wan, et al. J. Mater. Chem. C 5 (2017), 5316−5322.
[6] X. Chen, Y. Wang, H. Wang et al. Nano Energy 54 (2018), 280−287.
[7] This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 823717–ESTEEM3.