Quantitative objective-based ring TIRFM system calibration through back focal plane imaging
- Abstract number
- 1266
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.1266
- Corresponding Email
- [email protected]
- Session
- LSA.2 - Dynamic interactions in cells, organoids, tissue and entire organisms
- Authors
- Wenjie Liu (1), Cuifang Kuang (1)
- Affiliations
-
1. Zhejiang University
- Keywords
Total internal reflection fluorescence microscopy, Quantitative imaging, Back focal plane
- Abstract text
Although total internal reflection fluorescence microscopy (TIRFM) is a powerful tool for cell membrane-associated biological studies, there are still some limitations of TIRFM [1, 2]. The first one is the non-quantification of a TIRFM system. The crucial experimental parameters, such as the incident angle, are generally unknown, which also reduces the repeatability of the experiment. The second one is image artifacts, such as shadow and fringes, caused by the large-angle incidence of the light beam, which can be eliminated by using ring-shaped illumination (ring TIRFM), i.e., the incident light beam is controlled to scan at different azimuths under the same incident angle. Obviously, it poses a huge challenge to building a perfect ring TIRFM system, because the incident angle is difficult to be kept constant during the scan of a ring if it is completely dependent on manual system adjustment.
In light of these issues, here, we report a robust calibration approach to improve the quantification, repeatability, and precision of a ring TIRFM system, which can simultaneously calibrate the spinning illumination field (keeping the incident angle constant during the scan of a ring) and determine the incident angle by analyzing images acquired at the back focal plane (BFP) of an objective lens. The spinning field calibration is achieved in sequence by analyzing the BFP images acquired at different incident angles and azimuths, obtaining the critical angles at each azimuth, fitting the practical spinning shape, and then inputting the control system. The incident angle measurement is based on the aplanatic condition, i.e., the linear relationship of the radial position of the light focus at the BFP with the sine of the incident angle.
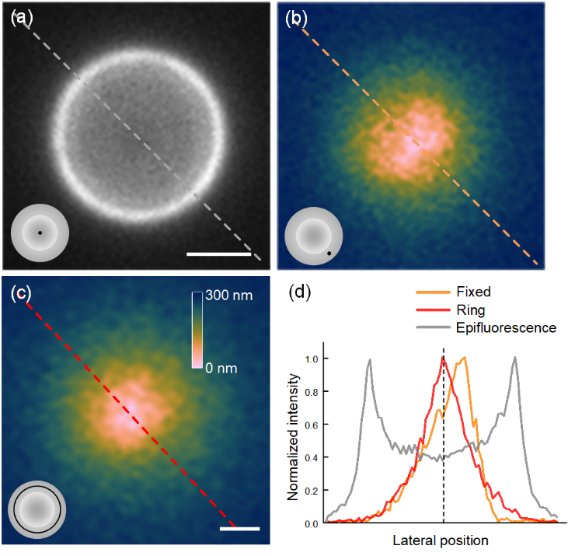
The effectiveness of our calibration routine is experimentally demonstrated by both the qualitative and quantitative comparison of the images acquired using different samples, illumination schemes, and calibration approaches. An experiment is presented in Figure 1, showing the influence of the illumination modes on the quantitative analysis of the TIRFM images. Comparing the pseudo-color depth maps calculated from raw fixed-azimuth TIRFM (Figure 1(b)) and ring TIRFM (Figure 1(c)) images, it can be found that Figure 1(b) has asymmetric depth distribution and off-center zero-depth point along the illumination direction, while Figure 1(c) has more symmetric and smooth depth distribution.
The advantages of our calibration approach include: 1) it is simple as it doesn’t require preparing special calibration samples or knowing any prior information; 2) it is robust enough to be unaffected by experiment differences, such as the inhomogeneity and polarity difference of samples; 3) it is fully automatic to avoid possible errors caused by manual measurement; 4) it is flexible and can be easily implemented on any objective-based TIRFM system. These characteristics should enable the calibration approach to greatly improve the practicability of TIRFM in life sciences.
Figure 1. The influence on the quantitative analysis of the TIRFM images using fixed-azimuth and ring-shaped illumination. The sample is a 5-μm microsphere coated with Alexa Fluor 488. (a) Epifluorescence image of the microsphere. (b, c) Depth distribution calculated from the raw fixed-azimuth TIRFM (b) and ring-TIRFM (c) images. The inset in the bottom-left corner of each figure represents the illumination scheme. (d) Normalized intensity profiles along the dashed lines shown in (a–c). Scale bars, 2 μm in (a) and 1 μm in (b, c).
- References
[1] J. A. Steyer, and W. Almers, "A real-time view of life within 100 nm of the plasma membrane," Nature reviews Molecular cell biology 2, 268 (2001).
[2] W. Liu, K. C. Toussaint Jr, C. Okoro, D. Zhu, Y. Chen, C. Kuang, and X. Liu, "Breaking the Axial Diffraction Limit: A Guide to Axial Super‐Resolution Fluorescence Microscopy," Laser & Photonics Reviews 12, 1700333 (2018).