Rapid 3D super resolution microscopy in live cell
- Abstract number
- 54
- Event
- European Microscopy Congress 2020 Invited Speakers
- DOI
- 10.22443/rms.emc2020.54
- Corresponding Email
- [email protected]
- Session
- LST.7 - Super-resolution microscopy
- Authors
- Ass. Prof. Ilaria Testa (1), Mr Andreas Bodén (1)
- Affiliations
-
1. KTH Royal Institute of Technology/Science for Life Laboratory
- Keywords
3D super-resolution fluorescence microscopy, switchable proteins, live super resolution, Invited Minisymposium speaker: Volumetric Imaging.
- Abstract text
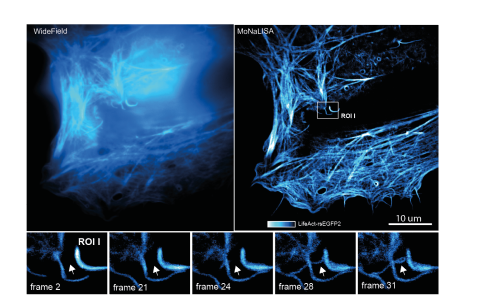
In recent years, the development of parallelized STED/RESOLFT super resolution microscopy techniques have shown impressive results combining diffraction unlimited lateral spatial resolution (< 70 nm) with increasingly fast acquisition speeds (~ 1 second) and optical sectioning. However, the diffraction-unlimited resolution improvement in these systems are always limited to the lateral dimension. Here we present a novel design of illumination patterns that allows for 3‑dimensional isotropic diffraction unlimited resolution while maintaining the rapid recording speed of a parallelized imaging system. Much like in the original RESOLFT-MoNaLISA set-up [1] the system is based on controlling the spatial distribution of ON‑OFF states in a sample labelled with reversibly switchable fluorescent proteins (RSFPs) [2]. Using RSFPs as labels allows for high resolution imaging at minimal light doses and is thus very suitable for live cell imaging. Designing illumination patterns that are modulated in all three spatial dimensions and combining these with the complete OFF-switching of RSFP allow to confine the emission spots in all the three spatial dimensions to sub-diffraction sized. The extended frequency content in the emission pattern manifests itself as isotropic diffraction unlimited resolution in the final images.
The spatial modulation in all three dimensions is made possible by the incoherent superposition of several independent illumination patterns, each pattern highly modulated in one spatial direction. Proper co-alignment of these patters results in sharply confined zero intensity volumes that, together with saturation of the fluorophore OFF-state, can create sub-diffraction sized emission volumes. Quantification and reassignment of the emitted photons from these volumes allows for reconstruction of the final isotropic super resolution images. We recorded 3D stacks across time of moving organelles and cytoskeleton in living human cells with spatial details < 100 nm in all the three dimensions.
- References
[1] Masullo L A, Boden A, Pennacchietti F, Coceano G, Ratz M and Testa I 2018 Enhanced photon collection enables four dimensional fluorescence nanoscopy of living systems Nature communications 9
[2] Pennacchietti F, Serebrovskaya E O, Faro A R, Shemyakina, II, Bozhanova N G, Kotlobay A A, Gurskaya N G, Boden A, Dreier J, Chudakov D M, Lukyanov K A, Verkhusha V V, Mishin A S and Testa I 2018 Fast reversibly photoswitching red fluorescent proteins for live-cell RESOLFT nanoscopy Nature methods 15 601-4