Tailoring of Shape and Size of Platinum Nanoparticles to Enhance Their Oxygen Reduction Reaction Performance
- Abstract number
- 1229
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.1229
- Corresponding Email
- [email protected]
- Session
- PSA.5 - Nanoparticles & Catalysts
- Authors
- Jan Michalička (2), Batyr Garlyyev (5), Johannes Fichtner (5), Sebastian Watzele (5), Kathrin Kratzl (1), Marlon Rück (1), Alessio Gagliardi (3), Wu Wang (4), Di Wang (4), Jan Macak (2), Aliaksandr Bandarenka (5, 1), Roland Fischer (1)
- Affiliations
-
1. Catalysis Research Center, Technical University of Munich
2. CEITEC - Brno University of Technology
3. Department of Electrical and Computer Engineering, Technical University of Munich
4. Institute of Nanotechnology, Karlsruhe Institute of Technology
5. Physics of Energy Conversion and Storage, Technical University of Munich
- Keywords
Oxygen reduction reaction, fuel cells, platinum, nanoparticles, transmission electron microscopy
- Abstract text
High oxygen reduction reaction (ORR) activity has been considered for many years as the key
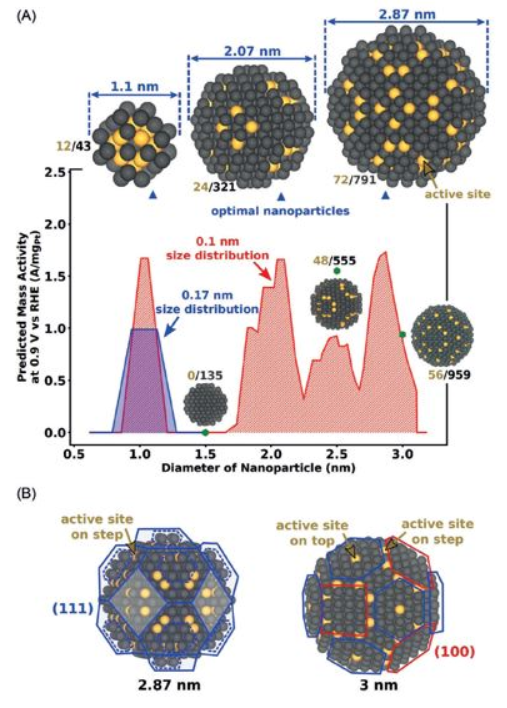
to many energy applications. Herein, we demonstrate two approaches how to exceptionally improve the ORR of platinum nanoparticles (NPs).The first approach is based on combining theory and experiment to prepare Pt NPs with optimal size for the efficient ORR [1]. Optimal nanoparticle sizes are predicted near 1, 2, and 3 nm by computational screening, Figure 1.
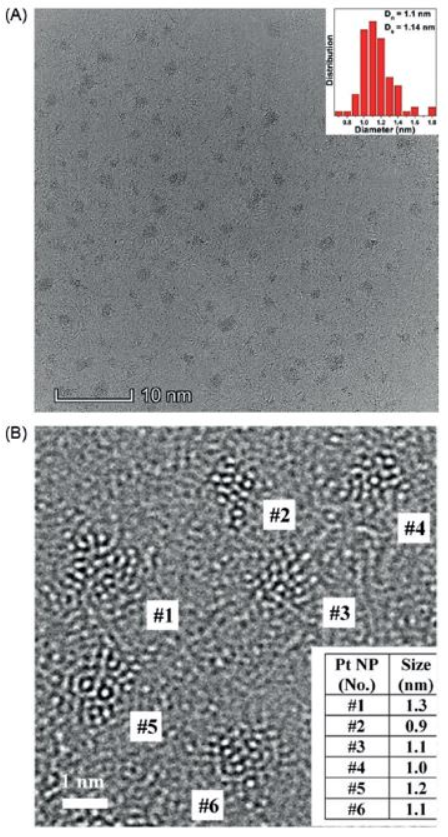
To corroborate our computational results, we have addressed the challenge of approximately 1 nm sized Pt nanoparticle synthesis with a metal–organic framework (MOF) template approach. The electrocatalyst structure will be demonstrated by a Cs-image corrected high-resolution transmission electron microscopy (HR-TEM), Figure 2. The measured mass activities (0.87 A/mgPt) are close to the computational prediction (0.99 A/mgPt) and it is the highest up to date mass activity among pure Pt catalysts for the ORR within similar size range [1, 2]. The specific and mass activities are twice as high as the Tanaka commercial Pt/C catalysis (0.42 A/mgPt) [3].
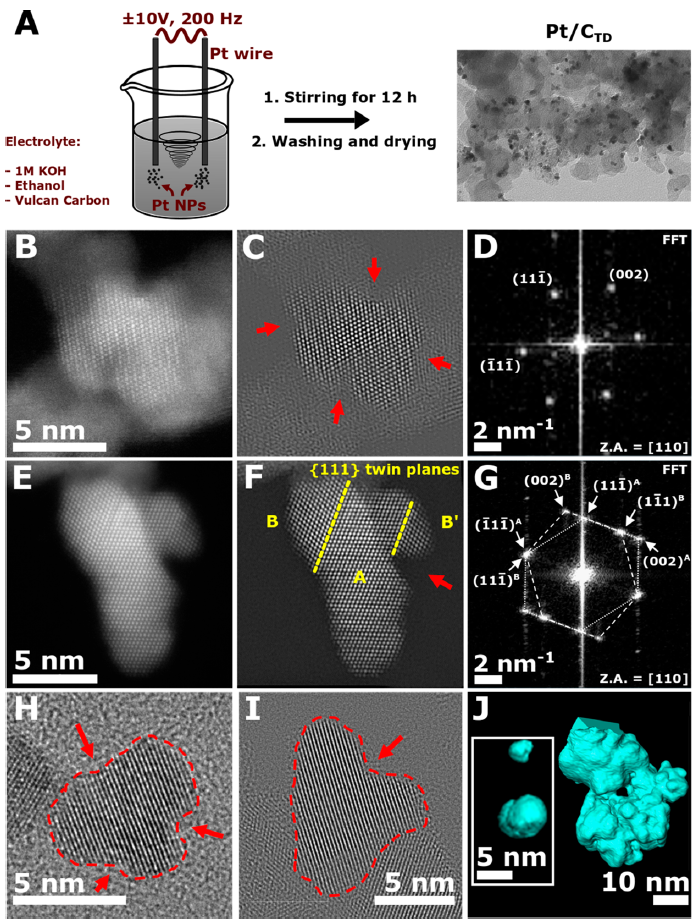
The second approach is based on theoretical considerations that Pt NPs with a degree of surface defects, e.g., containing surface concavities, can significantly enhance the catalytic activity towards the ORR. Hence, we developed a one-step and up-scalable top-down approach to produce Pt/C catalyst (with ~3 nm Pt NP diameter) starting from a bulk Pt wire [4]. The ORR activity of the developed catalyst exceeds that of the commercial Pt/C catalyst, ~2.7 times in terms of specific (1.62 mA/cm2Pt at 0.9 V vs the reversible hydrogen electrode) and ~1.7 times in terms of mass activity (0.71 A/mgPt) [5]. Besides, the technique used here reduces the complexity of the synthesis (and therefore production costs) compared to state-of-the-art bottom-up techniques. The evidence of a high density of surface defects (including the surface concavities) of Pt NPs will be demonstrated with use of the Cs-image corrected HR-TEM, high-resolution scanning transmission electron microscopy (HR-STEM) and also STEM tomography, with which the surface defects of the very small nanoparticles could be observed, Figure 3. [6].
Figure 1: A) Predicted mass activities plotted versus nanoparticle diameters. Optimal nanoparticles (blue triangles) are identified at diameters of 1.1 nm, 2.07 nm, and 2.87 nm. B) Low-index surfaces (111) (blue) and (100) (red) depicted on the 2.87 and 3 nm nanoparticle.
Figure 2: A) Overview HR-TEM image of Pt nanoparticles and size distribution histogram (inset). B) Detailed HR-TEM image shows the magnified image of six single Pt nanoparticles and the table shows their size.
Figure 3: (A) Schematic description of the synthetic procedure toward the Pt/CTD electrocatalyst. Application of an alternating sinusoidal potential (±10 V, 200 Hz) to Pt wires immersed in a suspension of Vulcan carbon in 1 M KOH and ethanol with vigorous stirring leads to the formation of carbon-supported Pt nanoparticles. (B, E) HR-STEM images of unsupported PtTD single nanoparticles with corresponding (C, F) Fourier filtered images and (D, G) FFT patterns (Z.A. = zonal axis). Certain concavities are highlighted by red arrows. Twin boundaries and twin grains A, B, B′are marked in (F). (H, I) HR-TEM images of unsupported PtTD nanoparticles. Concave regions are highlighted by red arrows. (J) 3D visualization of two unsupported, single PtTD nanoparticles, as well as a fraction of unsupported nanoparticle agglomerate, using STEM-HAADF tomography. Several concave regions are visible on both the individual and the agglomerated nanoparticles.
- References
[1] Garlyyev, B. et al., Angewandte Chemie, 28 (2019), p. 9596-9600
[2] Kratzl K. et al., J. Am. Chem. Soc., 141, 35 (2019), p. 13962-13969
[3] A. Orfanidi et al., J. Electrochem. Soc., 164 (2017), p. 418-426
[4] Fichtner, J. et al., ACS Appl. Mater. Interfaces, 11, 5 (2019), p. 5129-5135
[5] Fichtner, J. et al., ACS Catalysis, 10, XXX (2020), p. 3131-3142
[6] We gratefully acknowledge financial support from the TUM International Graduate School of Science and Engineering of Technical University of Munich (project number 11.01), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Grant No. 355784621 and under Germany’s excellence strategy EXC 2089/ 1−390776260, Germany’s excellence cluster “e-conversion”, DFG projects BA 5795/4-1 and BA 5795/3-1, and DFG Priority Program 1928 COORNETs. Support from CEITEC Nano Research Infrastructure and Karlsruhe Nano Micro Facility as well as financial support from the Ministry of Youth, Education and Sports of the Czech Republic, projects LM2015041 and LQ1601, are gratefully acknowledged. W.-J.L. gratefully acknowledges financial support from the Alexander von Humboldt fellowship for postdoctoral researchers.