Tem characterization of bimetallic nanocatalyst obtained by colloidal chemistry
- Abstract number
- 1011
- Event
- Virtual Early Career European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.1011
- Corresponding Email
- [email protected]
- Session
- PSA.5 - Nanoparticles & Catalysts
- Authors
- Cora Moreira da Silva (3), Frédéric Fossard (3), Ileana Florea (4), Mounib Bahri (2), Zakaria Halime (1), Armelle Girard (3, 5), Vincent Huc (1), Ovidiu Ersen (2), Ally Aukouloo (1), Annick Loiseau (3)
- Affiliations
-
1. Institut de Chimie Moléculaire et des Matériaux d’Orsay, CNRS, U. Paris-Saclay
2. Institut de Physique – Chimie des Matériaux de Strasbourg, CNRS, Université de Strasbourg
3. Laboratoire d'Etude des Microstructures, ONERA-CNRS, UMR104, U. Paris-Saclay
4. Laboratoire de Physique des Interfaces et des Couches Minces, École Polytechnique/CNRS
5. Université Versailles Saint-Quentin, U. Paris-Saclay
- Keywords
nanoalloys, colloidal synthesis, catalyzed reactions, in-situ microscopy, carbon solubility
- Abstract text
The reduced size of metallic nanoparticles (NPs) maximizes their specific surface area and their chemical reactivity for catalysis applications compared to their bulk counterpart [1]. Further playing with nanoalloyed particles provides additional keys for tuning catalytic properties beyond size effects. We have studied a new colloidal route towards the synthesis of nanoalloys with controlled size and chemical composition without phase segregation. Transmission Electron Microscopy techniques have been used to characterize properly these nanoalloys (STEM/EDX, diffraction, HR-TEM) and demonstrate their capabilities for deprotonation application (STEM/HAADF) or carbon nanotube growth (in-situ growth).
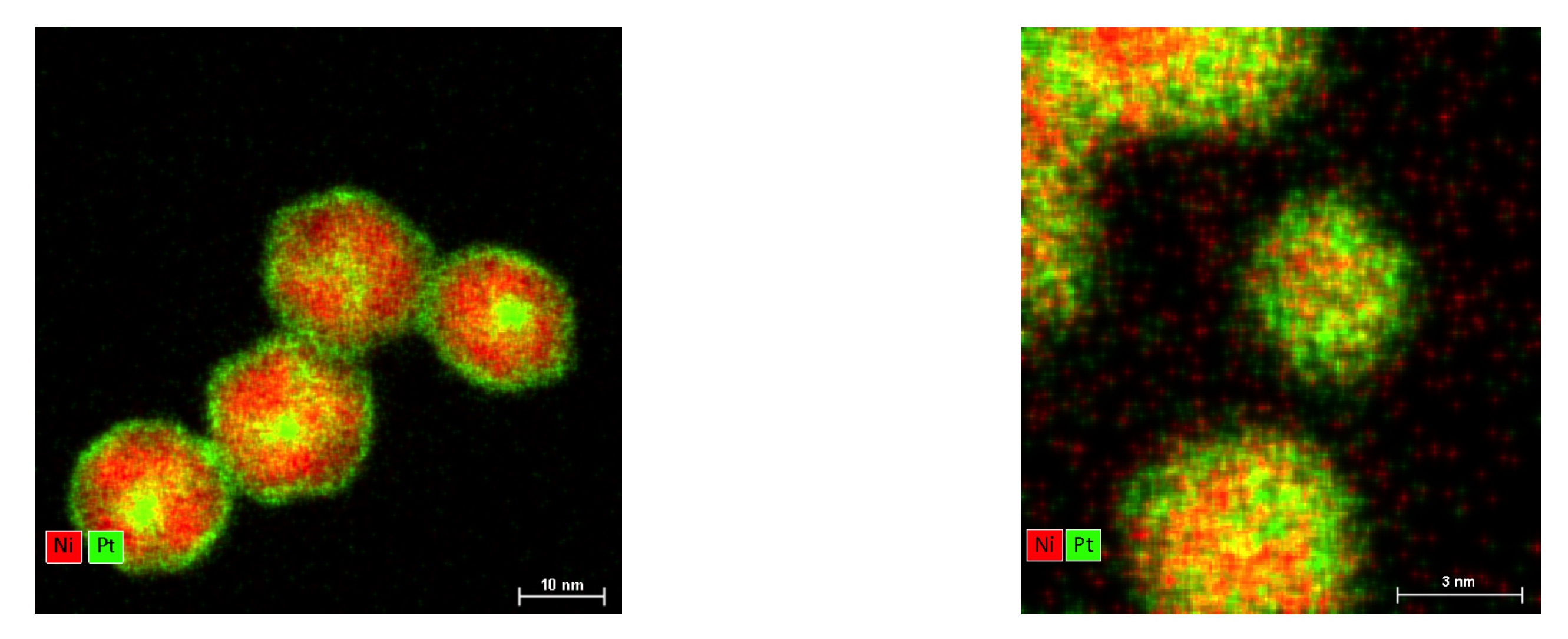
We report on the TEM characterization of nanocatalysts obtained by colloidal chemistry. The principle of the procedure is based on the correlation between oxidation-reduction potential of metal cations present in precursors and the required synthesis temperature to nucleate particle without phase segregation. We present results that illustrate the influence of the synthesis conditions (chemicals, temperature). The procedure is demonstrated on the synthesis of Face Centered Cubic (FCC) NixPt1−x nanoparticles as illustrated on fig 1. We used a corrected STEM to map the repartition of the elements within the particles and check that a solid solution has been achieved.
Figure 1 : STEM-HAADF image and chemical mapping using EDX with Pt (L line) in green and Ni (K line) in red of (on the left) Ni3Pt usual colloidal synthesis performed at 225 °C and (on the right) Ni3Pt synthesis by our route at high temperature.
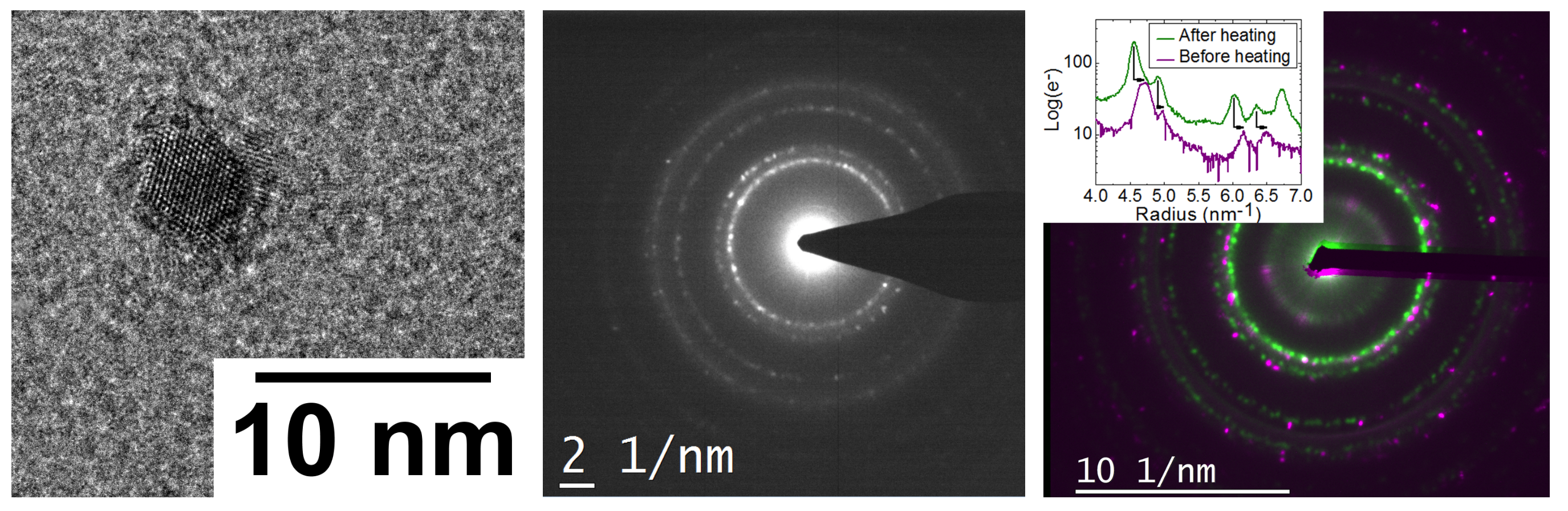
The structural characterization of the nanoparticles by HR-TEM and electron diffraction revealed that, regardless of the chemical elements, the FCC crystal system prevails and that the lattice parameter is larger than expected (see figure 2, and b) [2]. In order to get insights on the origin of this expansion, we also studied the influence of the surfactants interaction with the particle by in-situ electron microscopy (see figure 2,c ). As a matter of fact, the total/partial desorption of the surfactants results in a contraction of the lattice parameter which still remain larger than the bulk value. This highlights the traction force generated by the surfactants which can be adjusted by changing the surfactants type or in other word change the strength of the interaction with the NP outer atoms.
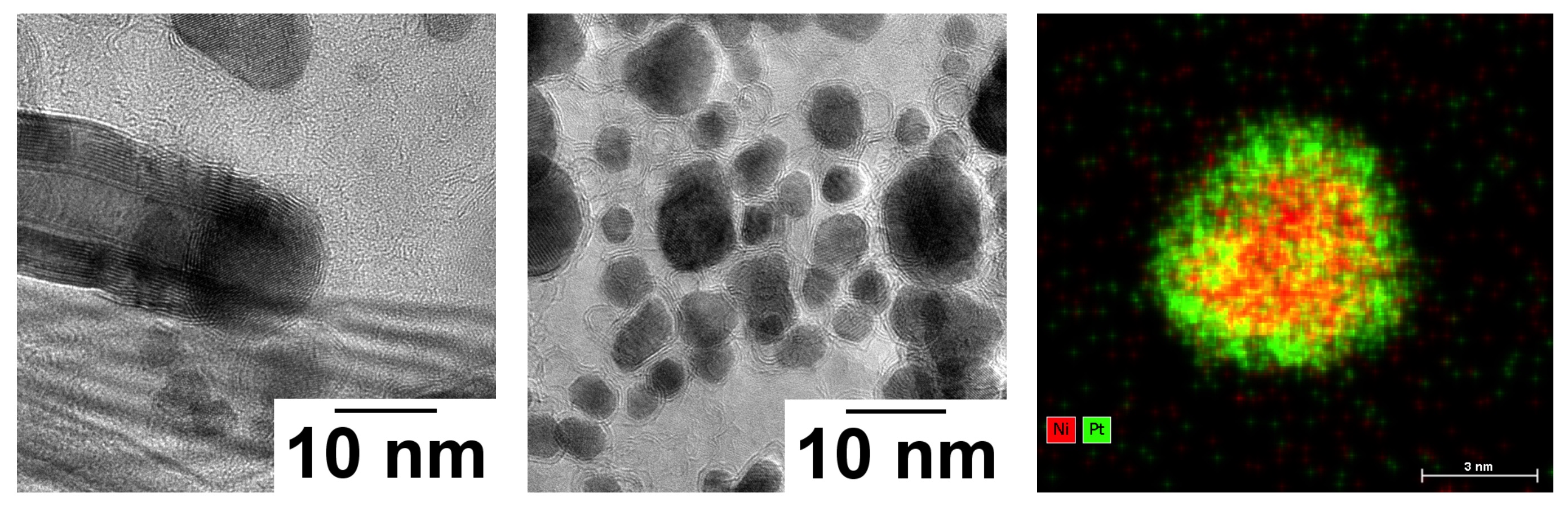
Figure 2 : from left to right : (a) STEM-HAADF micrography of Ni3Pt nanoparticles with (b) their electronic diffraction pattern showing FCC structure and a lattice parameter a = 0.377±0.005 nm. (c) Effect of heating on the lattice parameter of an assembly of Ni3Pt particles in sample : diffraction peaks are highlighted in green before heating and in purple after heating, showing a contraction of the lattice from a = 0.373 nm to a = 0.364 nm. In inset, intensity profiles of electron diffraction peaks before heating (green line) and after heating (purple line) showing the peaks shift.Among the properties that can tune by alloy effects, we choose to play with the carbon solubility of these nano-alloys by adjusting the Pt content in FexPt1−x from pure Fe to pure Pt. The C solubility in the particle is a key parameter to enhance the catalysis effect of the particle during the growth of CNT by chemical vapor deposition (CVD) [3]. Thanks to the NanoMAX microscope, we have been able to investigate the growth of CNT in the microscope using C2H2 as a C precursor and confirm the predictions. Figure 3 (a and b) shows an in-situ grown CNT with its particle. The growth mode appears to be tangential.
NixPt1-x system is widely studied in the catalyst community [4], Ni3Pt particles were tested for the water deprotonation reaction and analysed after several voltammetric cycles, phase segregation at the end of the reaction was observed (see fig. 3, c).
Figure 3 : From left to right : (a) Pur iron particles after carbon nanotube growth at 600 °C with C2H2 showing Fe2C structure at the base of the carbon nanotube, (b) FePt nanoparticles at the same condition showing no carbon nanotube growth nor an iron-carbide phase. (c) STEM-HAADF image and chemical mapping using EDX with Pt (L line) in green and Ni (K line) in red of Ni3Pt alloyed nanoparticles after the catalysis of a deprotonation reaction
In this work, we have developed a new colloidal procedure for the synthesis of bimetallic nanoalloys with controlled size and chemical composition. Our study demonstrates the versatility of this synthesis method along with the key role of temperature synthesis. Chemical composition and solid solution FCC structure of the nanoalloys are demonstrated, over the whole range of chemical compositions investigated, by combining different TEM imaging modes and electron diffraction techniques as well as EDX spectroscopy and mappings. We already checked the suitability of these new catalyst particles for CVD synthesis of CNT.
- References
[1] Cao, G.; Wang, Y. Nanostructures and Nanomaterials: Synthesis, Properties, and Applications; World Scientific series in nanoscience and nanotechnology; World Scientific, 2011.
[2] Xiong, S.; Qi, W.; Huang, B.; Wang, M. Size-, shape- and composition-dependent
alloying ability of bimetallic nanoparticles. Chem. Phys. Chem 2011, 12, 1317 -1324
[3] Aguiar-Hualde, J. M.; Magnin, Y.; Amara, H.; Bichara, C. Probing the role of carbon
solubility in transition metal catalyzing single-walled carbon nanotubes growth. Carbon
2017, 120, 226 - 232.
[4] Jurgens B.; Borchert H.; Ahrenstorf K.; Sonstrom P.; Pretorius A.; Schowalter M.; Gries K.; Zielasek V.; Rosenauer A.; Weller H.; Baumer M. Colloidally prepared nanoparticles for the synthesis of structurally well-defined and highly active heterogeneous catalysts, Angew. Chem. Int. Ed , 2008, 47, 8946 - 8949