Transformations of supported gold nanoparticles observed by in situ electron microscopy
- Abstract number
- 705
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.705
- Corresponding Email
- [email protected]
- Session
- PSA.5 - Nanoparticles & Catalysts
- Authors
- Pei Liu (4, 2), Tiantian Wu (1), Jacob Madsen (5, 3), Jakob Schiøtz (3), Jakob Birkedal Wagner (2), Thomas Willum Hansen (2)
- Affiliations
-
1. DTU Energy
2. DTU Nanolab
3. DTU Physics
4. EMAT
5. University of Vienna
- Keywords
Nanoparticles, Dynamics, Dynamics, In situ
- Abstract text
Oxide supported metal nanoparticles play an important role in heterogeneous catalysis. However, understanding the metal/oxide interface and their morphology and structure and how these change under a gaseous environment remains challenging. Herein, we investigate the interface between Au nanoparticles and a CeO2 substrate by environmental transmission electron microscopy with atomic resolution. We find that the Au nanoparticles have two preferential epitaxial relationships with the substrate, i.e. Type I (111)[−110]CeO2//(111)[−110]Au and Type II (111)[−110]CeO2//(111)[1−10]Au, where Type I is preferred. In situ observations in the presence of O2 show that the gas can stimulate the supported Au nanoparticles to transform between these two orientations even at room temperature [1]. Moreover, when increasing the temperature to 973 K, the transformation of an Au nanoparticle between the two orientation states and a non-crystalline state in the presence of O2 is also observed. DFT calculations of the binding energy? between Au and CeO2 in the two relationships is strongly influenced by the presence of oxygen vacancies. For a given position of a vacancy, there is a significant energy difference between the energy of the two types. However, for some positions, Type I is preferred, and for others, Type II, but the most favorable position of the vacancy for the two types has a very similar energy. This is consistent with the observation of both types of adhesion.
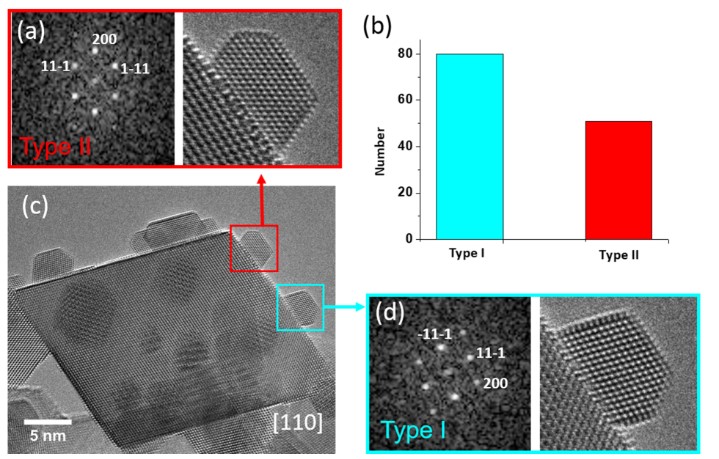
Figure 1(a) shows the typical morphology of a CeO2 supported Au nanoparticle on a model system. The shape of the commercial CeO2 support nanoparticles is normally octahedral with eight (111) surfaces. Two distinct crystalline orientation relationships between Au and the ceria support were observed. The distribution of these two types of relationships was measured on the model sample and was found to be 62% Type I and 38% Type II on 131 Au nanoparticles (Fig. 1(b)). The 25% lattice misfit that gives rise to a network of interfacial dislocations is formed to accommodate the strain that exists at the interface.
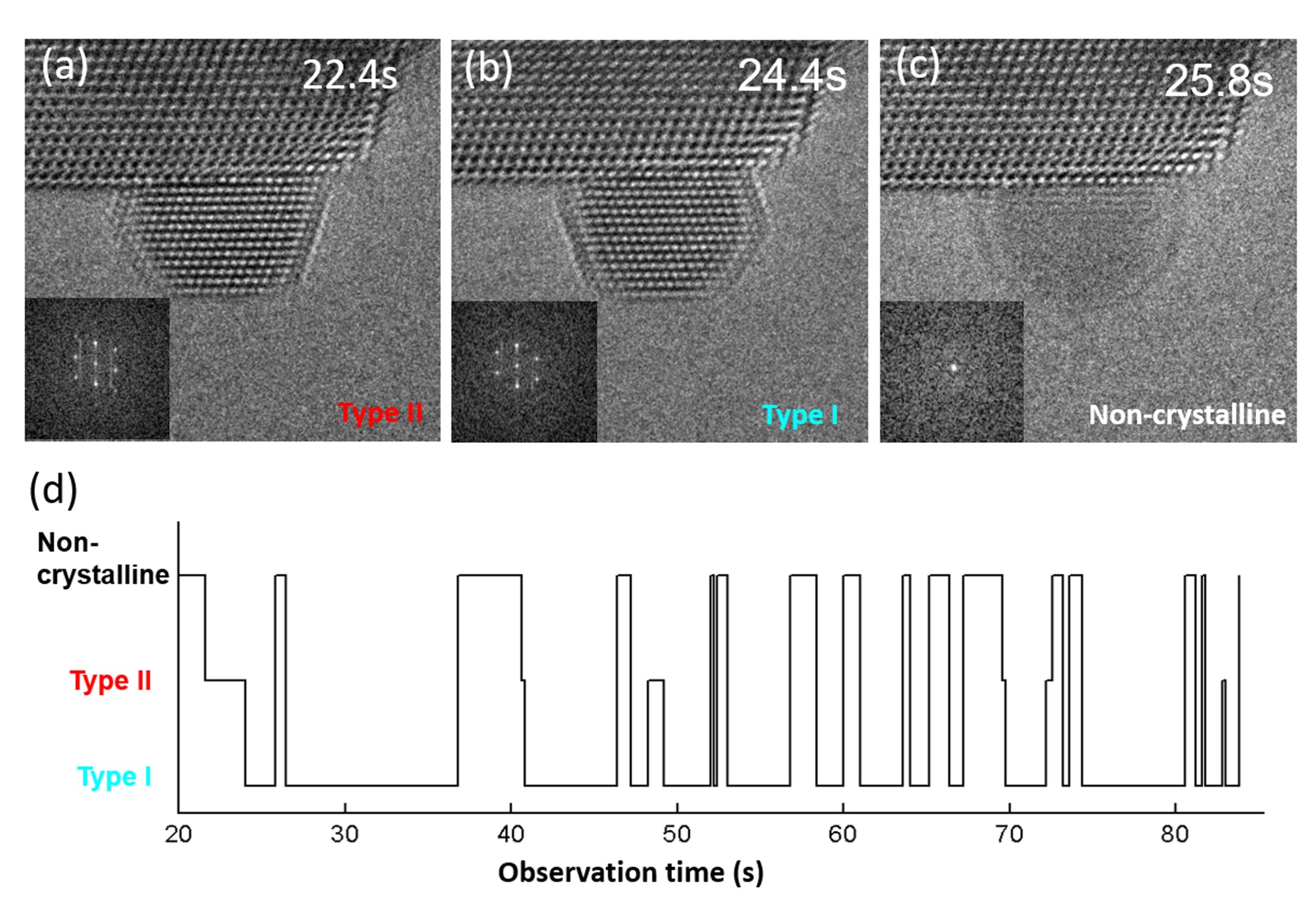
To obtain detailed information of the structural transformation process, image sequences of supported gold nanoparticles showing the transformations were acquired in situ during exposure to 4.5 Pa O2 at 973 K. Under these conditions, the nanoparticles change continuously between three structural states. Figures 2A, B, C show the structural transformation, which mainly occurs between the non-crystalline state, Type I and Type II. A timeline indicating the state of the particle is shown in Fig. 2D. The lifetime for Type II is shorter than that of Type I, which indicates a difference in energy of the two states.
This melting/resolidification phenomenon is suggested to be a result of the nanoscale size of the particles [2], the adsorption of oxygen on the surface [3] and the interaction with the CeO2 support [4]. All of these factors can give rise to a depression of the melting point of the Au nanoparticles.
Here, further measurements of the dynamics of the Au/CeO2 system along with analysis approaches will be discussed.
Figure 1: Typical morphology of the as-received Au/CeO2 sample along with the occurrence of the two states.
Figure 2: Different configurations of the ceria supported Au nanoparticles along with a timeline of the states.
- References
[1] P. Liu, T. T. Wu, J. Madsen et al., Nanoscale 11, (2019) 11885-11891.
[2] P. Schlexer, A. B. Andersen, B. Sebok et al., Part. Part. Syst. Charact. 36, (2019) 7.
[3] N. D. S. Canning, D. Outka, R. J. Madix, Surf. Sci. 141, (1984) 240-254.
[4] J. Lee, T. Tanaka, J. Lee et al., Calphad-Comput. Coupling Ph. Diagrams Thermochem. 31, (2007) 105-111.