Ultrastructural analysis of callitrichid hepatitis in captive marmosets and tamarins
- Abstract number
- 195
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.195
- Corresponding Email
- [email protected]
- Session
- LSA.7 - Pathology, immunocytochemistry and biomolecular labelling
- Authors
- Dr. Susanne Richter (1), Dr. Endre Sós (2), Dr. Angelika Auer (3), Dr. Zoltán Bagó (1)
- Affiliations
-
1. Austrian Agency for Health and Food Safety (AGES), Institute for Veterinary Disease Control Mödling
2. Budapest Zoo and Botanical Garden
3. Institute of Virology, University of Veterinary Medicine
- Keywords
arenavirus, callitrichid hepatitis, Lymphocytic choriomeningitis virus, marmosets, tamarins, zoonosis
- Abstract text
Introduction: Lymphocytic choriomeningitis virus (LCMV), a zoonotic Old World arenavirus [1] is widely distributed throughout Europe and America in the common house mouse (Mus musculus). Confirmatory evidence of LCMV in other parts of the world is lacking. Wild house mice are considered the primary source for human and animal infections. LCMV can be transmitted to humans and monkeys by contact with faeces or urine from infected rodents or with dust containing infective particles. LCMV infection of callitrichidae causes callitrichid hepatitis and was already published in the 1980/1990s [2,3,4]. Until now, ultrastructural investigations comprise in vitro infection studies of marmoset liver cell cultures [4] and experimental infection studies with ICR line-bred mice [5,6]. Here we present ultrastructural data on naturally acquired LCMV infections in callitrichids.
Material and Method: In 2019, several cases of sudden unexplained deaths were reported in captive callitrichids in the Budapest zoo. Four dead marmosets (Mico sp.) and two red-handed tamarins (Saguinus midas) were sent for pathomorphological investigations to the AGES-Institute for Veterinary Disease Control to investigate the cause of death. Fast negative staining technique (staining with 0,5% UA and 0,5% PTA) and ultrathin sectioning of EPON embedded tissue was chosen for subsequent ultrastructural analysis. Liver, lung, spleen, brain and faecal samples of the dead monkeys were analysed with a Zeiss TEM 906.
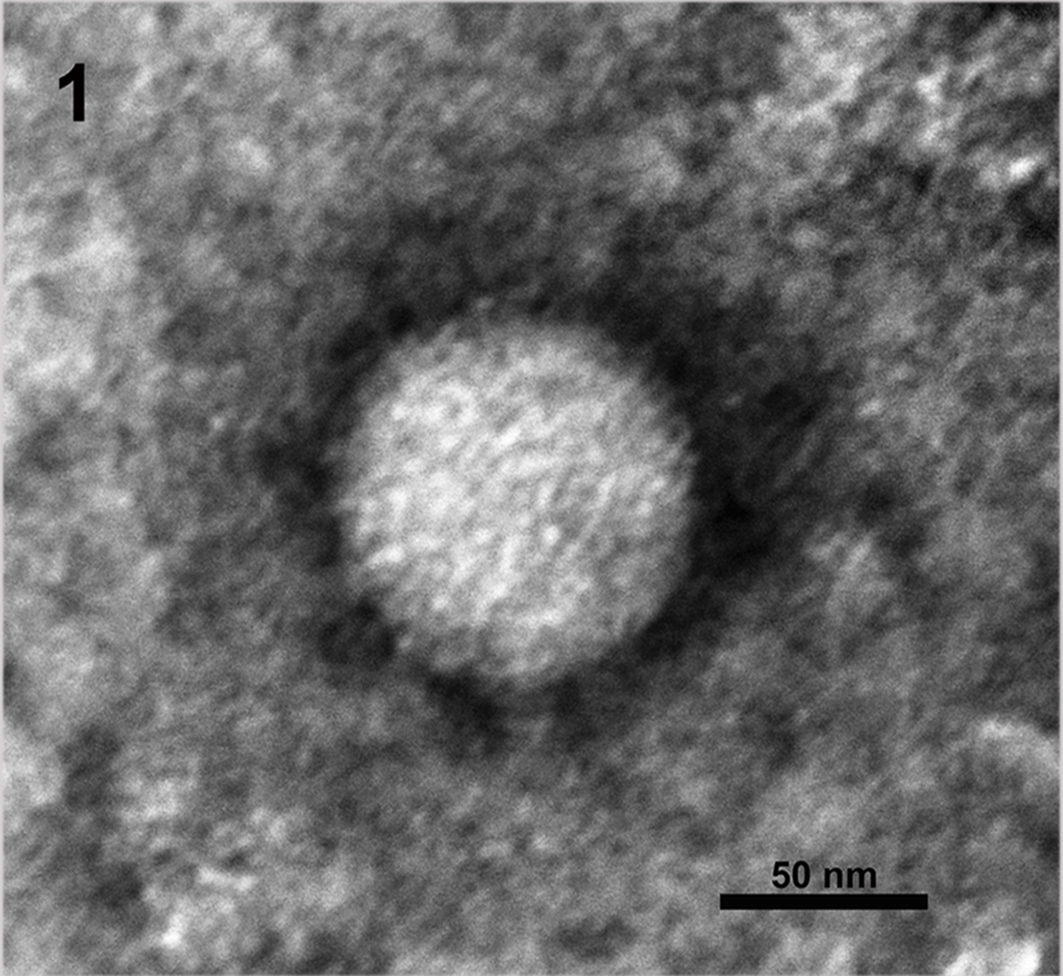
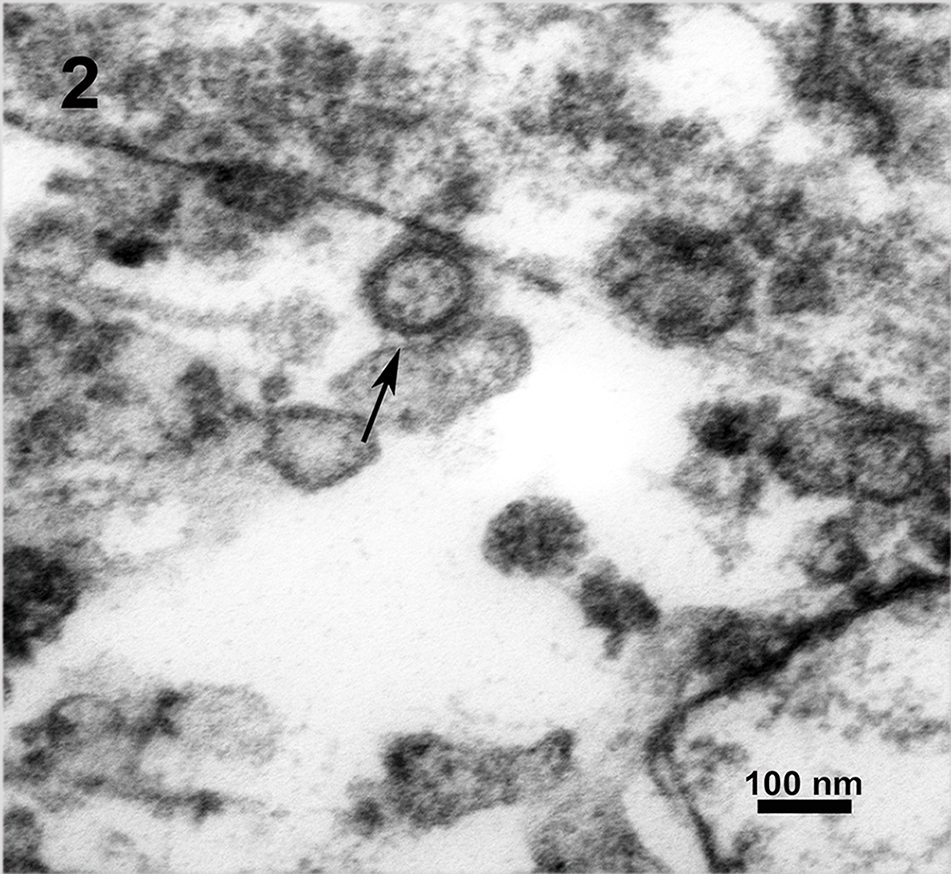
Results and Discussion: The most striking pathomorphological finding was a severe acute necrotising hepatitis with intracytoplasmatic inclusion bodies. Periportal infiltrates of lymphocytes and macrophages extended into the liver lobules. EM pictures showed LCMV particles in lung and liver of all monkeys. The virus particles had the typical structure of arenaviruses. They were enveloped, pleomorphic, 80 to 150 nm in diameter with a lipid bilayer envelope of approximately 10 to 15nm. Electron-dense internal ribosomal structures, typical for arenaviruses were inside the virions (Figure 2). Prominent inclusion bodies consisting of condensed ribosome-matrix masses persisted in lung cell as well as in liver cell cytoplasm. Arenaviruses were also detected in faecal samples (Figure 1). Additionally LCMV infection was confirmed by PCR.
Conclusions: Viremic mice within the enclosures were suggested to be the most likely source of LCMV infection of the investigated callitrichids. The potential exposure of many caretakers and zoo visitors to LCMV-infected tamarins and marmosets makes it particularly important to diagnose the infection with LCMV quickly.
Figure 1: Lymphocytic choriomeningitis virus (LCMV) found in the faecal samples of Mico sp. and Saguinus midas, negative staining, 60 000x
Figure 2: Utrathin EPON section of arenavirus infected liver tissue, LCMV (arrow), 27 800x
- References
[1] S R Radoshitzky et al, Arch. Virol. 2015, 160(7):1851-1874.
[2] V M Lucke, A M Bennett, Lab. Animals 1982,16:73-77.
[3] R J Montali et al, J. Infect. Dis. 1993,167(4):946-950.
[4] Ch B Stephensen et al, J. Virol. 1991, 65(8): 3995-4000.
[5] F A Murphy, S G Whitfield, Bull. World Health Organ. 1975, 52: 409-418.
[6] D H Walker et al, Exp. mol. Pathol. 1973, 23:245-265.