Ultrastructure analysis of plant tissue leaves of Brassica juncea L. and Tropaeolum majus L. by TEM
- Abstract number
- 1193
- Event
- Virtual Early Career European Microscopy Congress 2020
- Presentation Form
- Submitted Poster
- DOI
- 10.22443/rms.emc2020.1193
- Corresponding Email
- [email protected]
- Session
- LSA.2 - Dynamic interactions in cells, organoids, tissue and entire organisms
- Authors
- PhD student Ivana Vrca (1), Assistant professor Nives Kević (3), Assistant professor Ivana Restović (2), Full professor Ivana Bočina (3), Full professor Tea Bilušić (1)
- Affiliations
-
1. Faculty of Chemistry and Technology
2. Faculty of Philosophy
3. Faculty of Science
- Keywords
transmission electron microscopy (TEM), Brassica juncea L., Tropaeolum majus L., glucosinolates, myrosinase
- Abstract text
Introduction: Brown (Brassica juncea L.) mustard and Tropaeolum majus L. belong to Brassicaceae
family known for the presence of glucosinolates (GSLs), secondary plant metabolites. In the plant,
they coexist with an enzyme myrosinase, though separately stored in vacuoles of so-called S-cells
[1]. It was originally believed that the glucosinolate–myrosinase system in intact plants was stable
due to a spatial separation of the components. This has been coined as the ‘mustard oil bomb’ theory.
[2] GSLs and enzyme myrosinase come into contact when the plant tissue is damaged, releasing
unstable aglycones which spontaneously rearrange into a variety of reactive compounds like
thiocyanates, epithiontiriles, nitriles, oxazolidines and most important – isothiocyanates [1,2]. GSL
that is characteristic for Brassica juncea L. is sinigrin and for Tropaeolum majus L. is
glucotropaeolin. Sinigrin degrades and releases allyl isothiocyanate, while glucotropaeolin releases
benzyl isothiocyanate [3,4]. Some glucosinolate hydrolysis products act as a plant defence
compounds against insects and pathogens or have beneficial health effects on humans [2]. The aim
of this research was ultrastructure analysis of plant tissue leaves of Brassica juncea L. and
Tropaeolum majus L. and try to locate the components of the glucosinolate-myrosinase system
because it is different between plant organs and changes during a plant’s growth cycle.
Materials and methods: Samples were fixed with 2.5% solution of glutaraldehyde for 2 hours at
room temperature. Afterwards, samples were washed in PBS + 4% glucose, 3x15 min. Post-fixation
in 2% osmium tetroxide for 2 h and dehydration in ascending series of acetone (in each concentration
for 15 min) were made. Afterwards, samples were placed in a mixture of Spurr’s medium and acetone,
firstly in ¼ Spurr’s and ¾ acetone for 1 hour, then in ½ Spurr ’s and ½ acetone for 1 hour and lastly
in ¾ Spurr’s and ¼ acetone for 1 hour. This process was followed by placing the samples in
Spurr’smedium overnight in desiccator. Ultimately, samples were placed in a plastic mould and
polymerized in Spurr’s medium at 60 °C for 48 h (figure 1).
Utrathin sections (0.06 µm thick) were made and contrasted with 1% uranyl acetate and lead citrate.
Ultrathin sections were analysed by a transmission electron microscope JEM JEOL 1010, Japan at 80
kV.Figure 1. Preparated plant tissues for electron microscopy
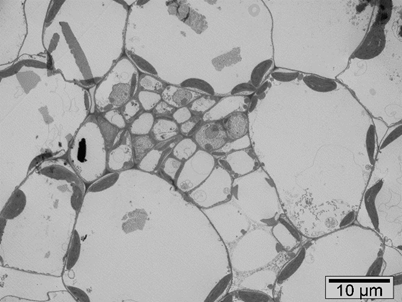
Results: Figure 2, 3 and 4 show tissue system of brown mustard and Tropaeolum majus L. nanum
where we can see chloroplasts, mitochondria, vacuoles, idioblasts, epidermis at the 2000x, 5000x and
8000x magnification. Kelly et al. (1998) analysed the cellular localization of glucosinolates in protein
bodies/vacuoles of Brassica juncea L. cotyledons from imbibed seeds. As to the subcellular
compartment in which glucosinolates are stored, the most potential place is vacuole [2]. The presence
of structurally defined myrosin cells in the phloem parenchyma was investigated in leaves.
Myrosinase can be found in idioblasts, also called myrosin cells. Idioblasts are specialized cells that
are scattered at low frequency and often as single cells among the other major cells in a tissue [5].Figure 2. Transverse section of brown mustard (TEM, 2000x)
Figure 3. Transverse section of brown mustard (TEM, 8000x)
Figure 4. Transverse section of Tropaeolum majus L. nanum (TEM, 5000x)
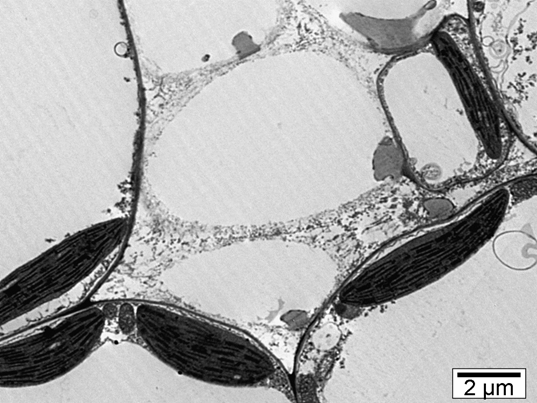
Figure 5 shows idioblastic phloem parenchyma cells which contain myrosinase. That cells are larger
and the staining was more granulated [5].
Figure 5. Myrosinase containing idioblastic phloem parenchyma cells in brown mustard (TEM, 8000x)
Conclusion: Plants from the Brassicaceae family contain glucosinolates whose breakdown products
show biological activities in human health such as anticancer, antimicrobial, antifungal and anti-
inflammatory, but also protect the plant itself from pests. To maximize the yield of desirable
degradation products such as isothiocyanates, we need to know the glucosinolate-myrosinase system
in plants that we use in our diet in everyday life and get the answer to the question how much it differs
from the system in the Arabidopsis thaliana model plant, which is so far the only well-researched
model plant.- References
[1] V. Boscaro et al, Molecules, 23 (11) (2018) 1-17.
[2] R. Kissen et al, Phytochem Rev 8 (2009) 69-86.
[3] M. Tian et al, American Journal of Dentistry, 26 (4) (2013) 180-184.
[4] H. J. Cho et al, Int. J. Mol. Sci. 17 (2016) 1-13.
[5] E. Andreason et al, Plant Physiology 127 (2001) 1750-1763.