Visualizing H2O Molecules Reacting at TiO2 Surface with TEM
- Abstract number
- 343
- Event
- European Microscopy Congress 2020
- DOI
- 10.22443/rms.emc2020.343
- Corresponding Email
- [email protected]
- Session
- PSA.5 - Nanoparticles & Catalysts
- Authors
- Prof. Yong Wang (1)
- Affiliations
-
1. Zhejiang University
- Keywords
Environmental TEM; TiO2; Water reaction
- Abstract text
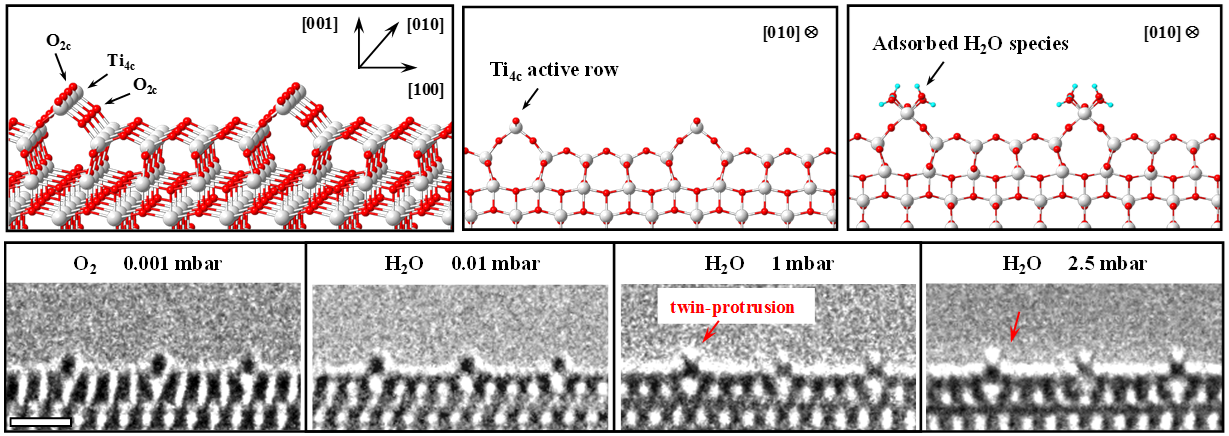
The advance of catalytic science and technology relies on the atomistic understanding of the reaction mechanism. It involves the identification of where and how the reactants adsorb on the surface, whether they react there, and what the intermediates are. In the past decades, significant progress has been achieved in characterizing the adsorption of molecules by scanning tunneling microscopy, or deducing the active sites through monitoring the variation of spectroscopy during the reaction. The most ideal way for understanding catalysis is, however, to “see” the reaction taking place at the molecular level. In this work, by employing anatase TiO2 (1×4)-(001) surface as catalyst, which provides highly ordered Ti4c “active rows”, we realized real-time monitoring of H2O molecule dissociating and reacting on the catalyst surface by in-situ environmental transmission electron microscopy [1-4]. The unique “twin-protrusion” configuration of adsorbed H2O was discovered (Fig. 1), which was visualized to react with CO on these active rows at the molecular level [1]. This work demonstrates that the in situ ETEM is capable to monitor the catalytic process taking place at highly ordered active sites.
Fig. 1 Adsorbed H2O species on TiO2 (001) surface (scale bar: 1nm)
- References
[1] W Yuan et al Science 367 (2020) 428.
[2] W Yuan et al Nano Lett. 16 (2016) 132
[3] W Yuan et al Chem. Mater. 29 (2017) 3189
[4] W Yuan et al Chem. Mater. 30 (2018) 288