Whole-Brain Biodistribution Analysis of Adeno-Associated Virus (AAV) with Single-Cell Resolution by Tissue Clearing and Light-Sheet Microscopy
- Abstract number
- 1145
- Event
- Virtual Early Career European Microscopy Congress 2020
- Presentation Form
- Submitted Oral
- DOI
- 10.22443/rms.emc2020.1145
- Corresponding Email
- [email protected]
- Session
- LST.10 - Lightsheet illumination/detection strategies to yield higher speed, higher resolution and higher throughput in Bioimaging
- Authors
- MSc Miguel Lopes (2), PhD Jacques Paysan (1), PhD. José Rino (3), PhD Student Sara Lopes (2, 4), PhD Rui Nobre (2, 4, 5), PhD Luísa Cortes (2), PhD Luís Pereira de Almeida (2, 5, 6)
- Affiliations
-
1. Carl Zeiss Microscopy GmbH
2. Center for Neuroscience and Cell Biology (CNC)
3. Instituto de Medicina Molecular, University of Lisbon
4. Institute for Interdisciplinary Research, University of Coimbra
5. ViraVector: Viral Vectors for Gene Transfer Core Facility, University of Coimbra
6. Faculty of Pharmacy, University of Coimbra
- Keywords
Adeno-Associated Virus
Gene therapy
Tissue Clearing
Light-Sheet Fluorescence Microscopy
- Abstract text
Gene therapy has emerged as a promising approach for treating a spectrum of neurodegenerative disorders by delivering healthy cargoes to the Central Nervous System. Of all gene therapy vectors, Adeno-Associated Viruses (AAVs) became a powerful system, transducing a wide range of cell types, by different routes of administration with an impressive safety profile [1]–[3].
However, a key challenge is the lack of suitable imaging technologies to efficiently monitor the tropism of AAVs and evaluate disease progression throughout the brain with single cell resolution. Traditional histological studies require sectioning and reconstruction to observe deep cellular structures with the disadvantages of being time consuming, difficult to automate, prone to tissue imperfections and disruption of many structures [4], [5].
Alternatively, recent studies turned their efforts to render whole organs optically transparent, thus overcoming obstacles imposed by light scattering, while preserving native cellular and molecular structure. Since the first attempts by Spalteholz more than a century ago [6], [7], a remarkable number of protocols have been developed and can be classified according to their main physical mechanism as: organic solvent-based, aqueous-based and tissue transformation methods [8]–[10]. Each clearing technique developed so far has its own advantages and drawbacks in terms of tissue opacity, size change, fluorescence preservation, time and cost efficiency.
Therefore, we aimed to assess a recently developed clearing protocol to evaluate AAV biodistribution in the mouse brain. For that, neonatal mice were intravenously injected with AAV9 encoding Green Fluorescence Protein (GFP), sacrificed 50 days later and the brains processed in parallel for immunohistochemistry (IHC) and clearing.
Cleared samples were imaged by Light-Sheet Fluorescence Microscopy (LSFM), an efficient method for high resolution imaging in wide and deep areas of tissues. We found that in short periods of time and without special and expensive equipment, this protocol renders highly cleared brains with minimal volume changes while simultaneously preserving GFP fluorescence.
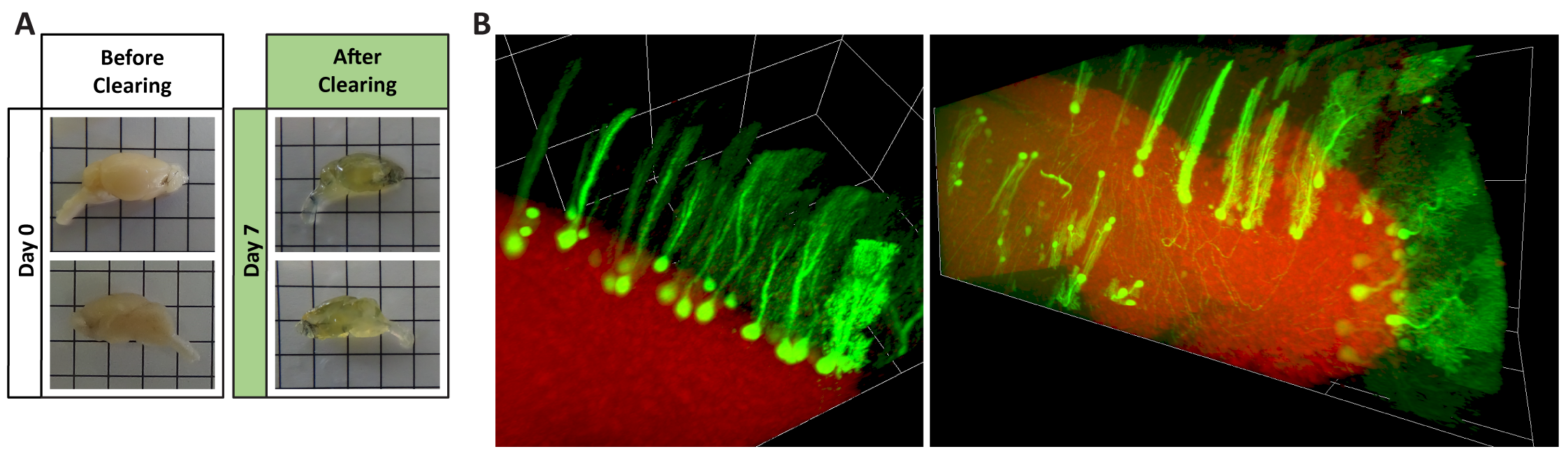
Figure 1 – Tissue clearing of mouse brain hemispheres. The tissue clearing protocol herein tested renders highly transparent brains in just seven days (A), while simultaneously preserving endogenous GFP fluorescence of Purkinje cells infected with AAV9 and DraQ5 stained nuclei (B).
Imaging of cleared entire brain hemispheres recapitulated the results obtained by traditional immunohistochemistry and showed that AAV9 can efficiently target and transduce several brain regions upon systemic administration, including the cerebellum, medulla, pons, midbrain, hippocampus and olfactory bulb. AAV9 seems to have a preferential tropism for Purkinje cells in the cerebellum and other neuronal cells, with little to no transduction of microglia, astrocytes and oligodendrocytes.
In conclusion, tissue clearing is conquering its way as a valuable tool in bioimaging by allowing three-dimensional visualization of biological structures that could not be seen in conventional methods. The new clearing protocol herein tested, when coupled to LSFM, takes the AAVs to a new level, proving its worth in tropism and gene therapy studies [11].
- References
[1] K. Miyake et al., “Serotype-independent method of recombinant adeno-associated virus (AAV) vector production and purification.,” J. Nippon Med. Sch., vol. 79, no. 6, pp. 394–402, 2012, doi: 10.1272/jnms.79.394.
[2] D. S. Ojala, D. P. Amara, and D. V Schaffer, “Adeno-Associated Virus Vectors and Neurological Gene Therapy.,” Neuroscientist, vol. 21, no. February 2014, pp. 84–98, Feb. 2014, doi: 10.1177/1073858414521870.
[3] D. W. Russell, I. E. Alexander, and A. D. Miller, “DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors.,” Proc. Natl. Acad. Sci. U. S. A., vol. 92, no. 12, pp. 5719–5723, Jun. 1995.
[4] J. R. Epp et al., “Optimization of CLARITY for Clearing Whole-Brain and Other Intact Organs,” eNeuro, vol. 2, no. 3, 2015, doi: 10.1523/ENEURO.0022-15.2015.
[5] Y. Isogai et al., “Optimized Protocol for Imaging Cleared Neural Tissues Using Light Microscopy.,” Methods Mol. Biol., vol. 1538, pp. 137–153, 2017, doi: 10.1007/978-1-4939-6688-2_11.
[6] W. Spalteholz, Über das Durchsichtigmachen von menschlichen und tierischen Präparaten, nebst Anhang: Über Knochenfärbung. Leipzig: S. Hirzel, 1911.
[7] W. Spalteholz, Über das Durchsichtigmachen von menschlichen und tierischen Präparaten und seine theoretischen Bedingungen : Nebst Anhang, Über Knochenfärbung. Leipzig: Verlag Von S. Hirzel, 1914.
[8] K. H. R. Jensen and R. W. Berg, “Advances and perspectives in tissue clearing using CLARITY.,” J. Chem. Neuroanat., vol. 86, pp. 19–34, Dec. 2017, doi: 10.1016/j.jchemneu.2017.07.005.
[9] J. Seo, M. Choe, and S.-Y. Kim, “Clearing and Labeling Techniques for Large-Scale Biological Tissues.,” Mol. Cells, vol. 39, no. 6, pp. 439–446, Jun. 2016, doi: 10.14348/molcells.2016.0088.
[10] L. Silvestri et al., “Clearing of fixed tissue: a review from a microscopist’s perspective.,” J. Biomed. Opt., vol. 21, no. 8, p. 81205, Aug. 2016, doi: 10.1117/1.JBO.21.8.081205.
[11] This work was funded by the ERDF through the Regional Operational Program Center 2020, Competitiveness Factors Operational Program (COMPETE 2020) and National Funds through FCT (Foundation for Science and Technology) – Imagene POCI-01-0145-FEDER-016807, BrainHealth2020 projects (CENTRO-01-0145-FEDER-000008), UID/NEU/04539/2019, PPBI (POCI-01-0145-FEDER-022122), ViraVector (CENTRO-01-0145-FEDER-022095), CortaCAGs (PTDC/NEU-NMC/0084/2014 | POCI-01-0145-FEDER-016719), SpreadSilencing POCI-01-0145-FEDER-029716, CancelStem POCI-01-0145-FEDER-016390, POCI-01-0145-FEDER-032309 as well as SynSpread, ESMI and ModelPolyQ under the EU Joint Program – Neurodegenerative Disease Research (JPND), the last two co-funded by the European Union H2020 program, GA No.643417; by National Ataxia Foundation (USA), the American Portuguese Biomedical Research Fund (APBRF) and the Richard Chin and Lily Lock Machado-Joseph Disease Research Fund.